
(a)
Interpretation:
The statement “Aniline is an
(a)
Answer to Problem 5MCP
The given statement is true.
Explanation of Solution
Aniline is the simplest aromatic amine with the formula
(b)
Interpretation:
The statement “Aniline has a lower boiling point than benzoic acid” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(b)
Answer to Problem 5MCP
The given statement is true.
Explanation of Solution
Aniline is an amine and benzoic acid is
The ability of primary and secondary amines to form
(c)
Interpretation:
The statement “Aniline is planar molecule” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(c)
Answer to Problem 5MCP
The given statement is false because it is slightly pyramidalized molecule.
Explanation of Solution
Aniline is slightly pyramidalized molecule, with hybridization of the nitrogen between
Hence, the given statement is false.
(d)
Interpretation:
The statement “The molecular formula of aniline is
(d)
Answer to Problem 5MCP
The given statement is false because the molecular formula of aniline is
Explanation of Solution
Aniline is the simplest aromatic amine with the formula
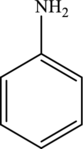
Hence, the statement “Aniline has a molecular formula of
(e)
Interpretation:
The statement “Aniline is a structural isomer of xylene” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(e)
Answer to Problem 5MCP
The given statement is false because both aniline and xylene have different molecular formulas.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of aniline is
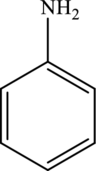
The molecular formula of xylene is
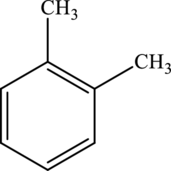
Both these are not structural isomers with each other.
Hence, the statement “aniline is structural isomer of xylene” is false because they have different molecular formulas.
(f)
Interpretation:
The statement “Aniline is more soluble than phenol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(f)
Answer to Problem 5MCP
The given statement is false because both aniline and phenol are sparingly soluble in water.
Explanation of Solution
Aniline does not form hydrogen bonds with water to a very large extent due to the presence of large hydrophobic
The hydroxyl group of phenol is polar in nature, the oxygen is more electronegative than hydrogen, and hence it attracts shared electrons and acquires partial negative charge. The hydrogen of hydroxyl group in phenol carries partial negative charge. Phenol forms hydrogen bonding with water. However, the large part of phenol molecule is phenyl group is non-polar, thus making it sparingly soluble in water.
Hence, the given statement is false because both aniline and phenol are sparingly soluble in water.
(g)
Interpretation:
The statement “Aniline produces phenylammonium ion in water” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(g)
Answer to Problem 5MCP
The given statement is true.
Explanation of Solution
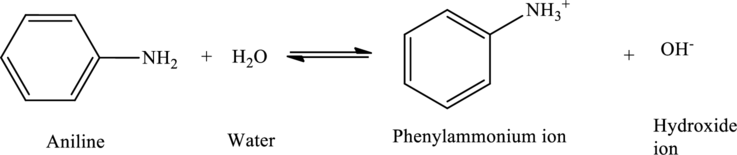
Aniline acts as bases, accepting
Want to see more full solutions like this?
Chapter 15 Solutions
GENERAL ORG+BIOCHEMISTRY CONNECT ONLY
- Hello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forwardCite the stability criteria of an enamine..arrow_forwardCalculate the pH of a 0.01m solution of acetic acid use pka of 4.75arrow_forward
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forward
- Please answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forward
- Consider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





