
(a)
Interpretation:
The statement “
(a)
Answer to Problem 3MCP
The given statement is false because amides shows a presence of strong electronegative oxygen atom of carboxyl group causes a very strong attraction between the lone pair of nitrogen electrons and the carbonyl group. Because, of which the unshared pair of electrons cannot hold a proton.
Explanation of Solution
Amide shows a presence of strong electronegative oxygen atom of carboxyl group causes a very strong attraction between the lone pair of nitrogen electrons and the carbonyl group. Because, of which the unshared pair of electrons cannot hold a proton.
Hence,
(b)
Interpretation:
The statement “
(b)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Amides have highest melting point when compared to alcohols and
The ability of primary and secondary amines to form
Hence,
(c)
Interpretation:
The statement “
(c)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(d)
Interpretation:
The statement “
(d)
Answer to Problem 3MCP
The given statement is false because
Explanation of Solution
The structure of
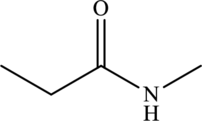
The carbonyl group present in
The statement “
(e)
Interpretation:
The statement “
(e)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
The structure of
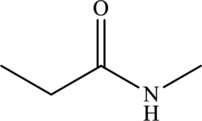
From the structure of
Hence, the given statement is true.
(f)
Interpretation:
The statement “
(f)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Amides have highest melting point when compared to
Hence, the given statement is true.
(g)
Interpretation:
The statement “
(g)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(h)
Interpretation:
The statement “
(h)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(i)
Interpretation:
The statement “
(i)
Answer to Problem 3MCP
The given statement is false because it comprises of carbonyl functional group.
Explanation of Solution
Carboxyl group consists of carbonyl group and hydroxyl group.
The structure of
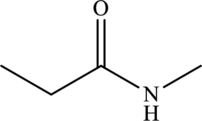
(j)
Interpretation:
The statement “
(j)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of
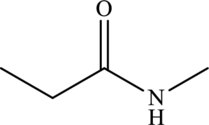
The molecular formula of
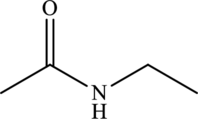
Both these are structural isomers with each other and hence, the statement “
Want to see more full solutions like this?
Chapter 15 Solutions
GEN ORG/BIO CHEM LL W/CONNECT ACCESS
- X 5 Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. CI Br Br Br 0 None of these molecules have a total of five ẞ hydrogens. Explanation Check esc F1 F2 tab caps lock fn Q @2 A W # 3 OH O OH HO © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility IK F7 F7 F8 TA F9 F10 & 6 28 * ( > 7 8 9 0 80 F3 O F4 KKO F5 F6 S 64 $ D % 25 R T Y U பட F G H O J K L Z X C V B N M H control option command P H F11 F12 + || { [ command optionarrow_forwardAn open vessel containing water stands in a laboratory measuring 5.0 m x 5.0 m x 3.0 m at 25 °C ; the vapor pressure (vp) of water at this temperature is 3.2 kPa. When the system has come to equilibrium, what mass of water will be found in the air if there is no ventilation? Repeat the calculation for open vessels containing benzene (vp = 13.1 kPa) and mercury (vp = 0.23 Pa)arrow_forwardEvery chemist knows to ‘add acid to water with constant stirring’ when diluting a concentrated acid in order to keep the solution from spewing boiling acid all over the place. Explain how this one fact is enough to prove that strong acids and water do not form ideal solutions.arrow_forward
- The predominant components of our atmosphere are N₂, O₂, and Ar in the following mole fractions: χN2 = 0.780, χO2 = 0.21, χAr = 0.01. Assuming that these molecules act as ideal gases, calculate ΔGmix, ΔSmix, and ΔHmix when the total pressure is 1 bar and the temperature is 300 K.arrow_forwarddG = Vdp - SdT + μA dnA + μB dnB + ... so that under constant pressure and temperature conditions, the chemical potential of a component is the rate of change of the Gibbs energy of the system with respect to changing composition, μJ = (∂G / ∂nJ)p,T,n' Using first principles prove that under conditions of constant volume and temperature, the chemical potential is a measure of the partial molar Helmholtz energy (μJ = (∂A / ∂nJ)V,T,n')arrow_forwardThe vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forward
- Draw the structure of A, the minor E1 product of the reaction. Cl Skip Part Check F1 esc CH_CH OH, D 3 2 Click and drag to start drawing a structure. 80 R3 F4 F2 F3 @ 2 # $ 4 3 Q W 95 % KO 5 F6 A F7 × G ☐ Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ►II A A F8 F9 F10 FL 6 7 88 & * 8 9 LLI E R T Y U A S D lock LL F G H 0 P J K L Z X C V B N M 9 Harrow_forwardFrom the choices given, which two substances have the same crystal structure? (Select both) Group of answer choices ZnS (zincblende) Diamond TiO2 (rutile) ZnS (wurtzite)arrow_forwardPotassium (K) blends with germanium (Ge) to form a Zintl phase with a chemical formula of K4Ge4. Which of the following elements would you expect potassium to blend with to form an alloy? Electronegativities: As (2.0), Cl (3.0), Ge (1.8), K (0.8), S (2.5), Ti (1.5) Group of answer choices Arsenic (As) Sulfur (S) Chlorine (Cl) Titanium (Ti)arrow_forward
- Consider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forwardHow many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forwardV Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





