
(a)
Interpretation:
The statement “
(a)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
Alcohols have strong intermolecular hydrogen bonding. The
On the other hand, the polarity of the amine nitrogen is less than the oxygen in alcohol. The nitrogen amine is less electronegative than oxygen in the alcohol. Therefore, the dipole on
(b)
Interpretation:
The statement “
(b)
Answer to Problem 1MCP
The given statement is false because of the presence of strong intermolecular hydrogen bonding in octanoic acid, it has higher boiling point than
Explanation of Solution
Carboxylic acids have strong intermolecular hydrogen bonding with each other and strong dipole-dipole attractions. The presence of polar carboxyl group and intermolecular hydrogen bonding makes these acids have higher boiling points. The presence of dimers increases the strength of the van der Waals dispersion forces, which makes them have high boiling points.
The ability of primary and secondary amines to form
Hence, the statement “
(c)
Interpretation:
The statement “
(c)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
Amine with five or fewer carbon atoms is soluble in water.
(d)
Interpretation:
The statement “
(d)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
In
(e)
Interpretation:
The line formula of
(e)
Answer to Problem 1MCP
The given line formula of
Explanation of Solution
The given line formula is,

The above line formula represents
(f)
Interpretation:
The statement “
(f)
Answer to Problem 1MCP
The given statement is false because
Explanation of Solution
(g)
Interpretation:
The statement “
(g)
Answer to Problem 1MCP
The given statement is false because it secondary amine.
Explanation of Solution
The structure of

From the structure of
(h)
Interpretation:
The statement “
(h)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
Reduction of amides in the presence of
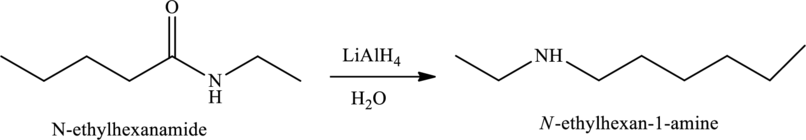
(i)
Interpretation:
The statement “
(i)
Answer to Problem 1MCP
The given statement is false because they have different structural and molecular formulas.
Explanation of Solution
The structure of
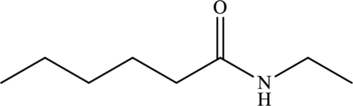
The structure of

(j)
Interpretation:
The statement “The reaction of
(j)
Answer to Problem 1MCP
The given statement is false because the neutralization reaction of the
Explanation of Solution
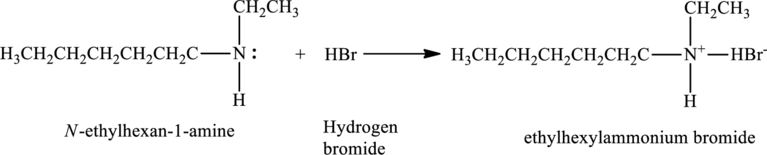
Hence the given statement is false because the neutralization reaction of
(k)
Interpretation:
The statement “The alkyl ammonium salt would be more water soluble than the amine” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(k)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
By adding a strong acid to a water-insoluble amine, a water-soluble alkylammonium salt can be formed. The salt could be converted back to an amine by reaction with strong base.
Alkyl ammonium salts can neutralize hydroxide ions. Water is formed and the protonated amine cation is converted into an amine. The nitrogen atom of an ammonium salt has a positive charge, alkyl ammonium salts are more water-soluble than amines.
(l)
Interpretation:
The statement “
(l)
Answer to Problem 1MCP
The given statement is false because it is not a cyclic compound.
Explanation of Solution
The structure of

Want to see more full solutions like this?
Chapter 15 Solutions
General, Organic, and Biochemistry
- Draw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forward
- Indicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forwardHow many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.arrow_forward
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





