
(a)
Interpretation:
The given compound can exhibit optical activity. The chiral centres have to be identified in the compound.
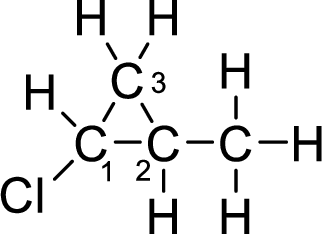
Concept Introduction:
The isomers which are mirror images of each other and are non-superimposable are said to be optical isomers and the isomerism is said to be optical isomerism. The optical isomers are also called as enantiomers. The optical isomers which rotates the plane-polarized light are optically active.
The optical isomers are asymmetric and plane of symmetry will be absent. An organic molecule is said to be chiral when it is bonded to four different groups and is asymmetric. The carbon atom bonded to four different groups is said to be chiral carbon or asymmetric carbon. An organic compound is said to be optically active when it contains at least one chiral center.
(b)
Interpretation:
The given compound can exhibit optical activity. The chiral center has to be identified in the compound.
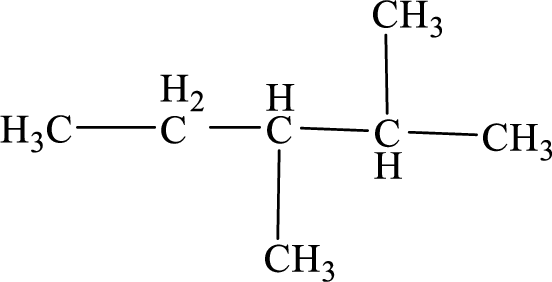
Concept Introduction:
The isomers which are mirror images of each other and are non-superimposable are said to be optical isomers and the isomerism is said to be optical isomerism. The optical isomers are also called as enantiomers. The optical isomers which rotates the plane-polarized light are optically active.
The optical isomers are asymmetric and plane of symmetry will be absent. An organic molecule is said to be chiral when it is bonded to four different groups and is asymmetric. The carbon atom bonded to four different groups is said to be chiral carbon or asymmetric carbon. An organic compound is said to be optically active when it contains at least one chiral center.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
CHEMISTRY THE MOLECULAR NATURE OF MATTER
- For a CARS experiment on a Raman band 918 cm-1, if omega1= 1280 nm, calculate the omega2 in wavelength (nm) and the CARS output in wavelength (nm).arrow_forwardI need help with the following questionarrow_forwardFor CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forward
- Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forward
- Can you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





