
(a)
The temperature and pressure at point
(a)
Answer to Problem 13P
The pressure at point
Explanation of Solution
There is
The cycle of the ideal gas is shown below in figure 1.
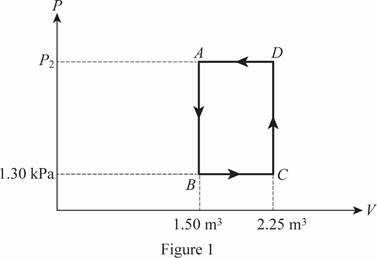
The pressure at point
Write the formula for the pressure at point
Here,
Write the formula for the temperature at point
Here,
Conclusion:
Substitute
Substitute
The pressure at point
(b)
The net work done on the gas if the gas completes 4 cycles.
(b)
Answer to Problem 13P
The net work done on the gas if the gas completes 4 cycles is
Explanation of Solution
There is
The cycle of the ideal gas is shown below in figure 1.
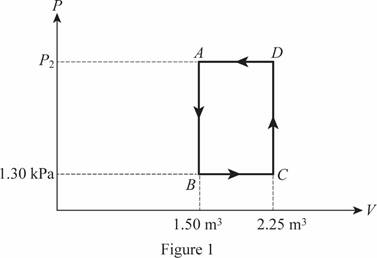
The pressure at point
Write the formula for the work done in four cycle of the gas.
Here,
Conclusion:
Substitute
The net work done on the gas if the gas completes 4 cycles is
(c)
The internal energy of the gas at point
(c)
Answer to Problem 13P
The internal energy of the gas at point
Explanation of Solution
There is
The cycle of the ideal gas is shown below in figure 1.

The pressure at point
Write the formula for the internal energy at point
Here,
Conclusion:
Substitute
The internal energy of the gas at point
(d)
The total change in internal energy of the gas during the four cycles.
(d)
Answer to Problem 13P
There is no change in internal energy of the gas through the complete four cycles.
Explanation of Solution
There is
The pressure at point
For ideal gases, change in internal energy depends on the change in temperature only. If the temperature remains same, there is no change in internal energy.
Since in the course of the complete four cycles of the gas, there is no change in temperature of the gas, there is no change in the internal energy.
Conclusion:
There is no change in internal energy of the gas through the complete four cycles.
Want to see more full solutions like this?
Chapter 15 Solutions
Physics
- Pls help ASAParrow_forward14. A boy is out walking his dog. From his house, he walks 30 m North, then 23 m East, then 120 cm South, then 95 m West, and finally 10 m East. Draw a diagram showing the path that the boy walked, his total displacement, and then determine the magnitude and direction of his total displacement.arrow_forwardPls help ASAParrow_forward
- 12. A motorboat traveling 6 m/s, West encounters a water current travelling 3.5 m/s, South. a) Draw a vector diagram showing the resultant velocity, then determine the resultant velocity of the motorboat. b) If the width of the river is 112 m wide, then how much time does it take for the boat to travel shore to shore? c) What distance downstream does the boat reach the opposite shore?arrow_forwardLake Erie contains roughly 4.00⋅10114.00⋅1011 m3 of water. Assume the density of this water is 1000. kg/m3 and the specific heat of water is 4186 J/kg˚C. It takes 2.31x10^19 J of energy to raise the temperature of that volume of water from 12.0 °C to 25.8 ˚C. An electric power plant can produce about 1110 MW. How many years would it take to supply this amount of energy by using the 1110 MW from an electric power plant?arrow_forwardPls help ASAParrow_forward
- Pls help ASAParrow_forwardm m $2° 15. A truck is stopped at a red light. Once the light turns green, the truck accelerates forward at 1.75- that same instant, a car moving with a constant speed of 50 — passes the truck. a) How many seconds will it take for the truck to catch up to the car? S b) How many metres will the truck travel before it catches up to the car? Atarrow_forwardPls help ASAParrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





