
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059230
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 52TE
What do the compounds cyclopropane and propene have in common?
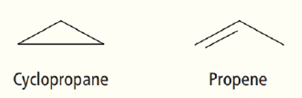
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A capacitor with a capacitance of C = 5.95×10−5 F is charged by connecting it to a 12.5 −V battery. The capacitor is then disconnected from the battery and connected across an inductor with an inductance of L = 1.55 H .
(D)What is the charge on the capacitor 0.0235 s after the connection to the inductor is made? Interpret the sign of your answer. (e) At the time given in part (d), what is the current in the inductor? Interpret the sign of your answer. (f) Atthe time given in part (d), how much electrical energy is stored in the capacitor and how much is stored in the inductor?
Close-up view
etermine;
The volume of the object given that the initial level of water in the measuring cylinder
23cm3.
The density of the object.
simple cell made by dipping copper and zinc plates into dilute sulfuric acid solution. A bull
onnected across the plates using a wire.
State what constitute current flow through the wire
The bulb connected across is observed to light for some time and then goes out. State t
possible
asons for this observation.
State two ways in which the processes named in question (b) above can be minimized t
the bulb light for a longer period.
ead
is rated 80Ah. Determine the current that can be drawn continuously
Answers with -1.828, -1.31 or 939.3 are not correct.
Chapter 14 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 14 - How do two structural isomers differ from each...Ch. 14 - How do two structural isomers similar to each...Ch. 14 - What physical property of hydrocarbons is used in...Ch. 14 - What types of hydrocarbons are more abundant in...Ch. 14 - To how many atoms is a saturated carbon atom...Ch. 14 - What is the difference between a saturated...Ch. 14 - How many multiple bonds must a hydrocarbon have in...Ch. 14 - Aromatic compounds contain what kind of ring?Ch. 14 - What is a heteroatom?Ch. 14 - Why do heteroatoms make such a difference in the...
Ch. 14 - How is a heteroatom related to a functional group?Ch. 14 - Why are low-formula-mass alcohols soluble in...Ch. 14 - What distinguishes an alcohol from a phenol?Ch. 14 - What distinguishes an alcohol from an ether?Ch. 14 - Which hetroatom is characteristic of an amine?Ch. 14 - Do amines tend to be acidic, neutral, or basic?Ch. 14 - Are alkaloids found in nature?Ch. 14 - What are some examples of alkaloids?Ch. 14 - Which elements make up the carbonyl group?Ch. 14 - How are ketones and aldehydes related to each...Ch. 14 - How are amides and carboxylic acids related to...Ch. 14 - From what naturally occurring compound is aspirin...Ch. 14 - What happens to the double bond of a monomer that...Ch. 14 - What is released in the formation of a...Ch. 14 - Why is plastic wrap made of polyvinylidene...Ch. 14 - Prob. 26RCCCh. 14 - In the lock-and-key model, is a drug viewed as the...Ch. 14 - What holds a drug to its receptor site?Ch. 14 - Which fits better into the opioid receptor...Ch. 14 - How does the effect of a drug wear off?Ch. 14 - Prob. 34TCCh. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Prob. 39TECh. 14 - What property of carbon allows for the formation...Ch. 14 - Prob. 41TECh. 14 - Draw all the structural isomers for hydrocarbons...Ch. 14 - How many structural isomers are shown here? .Ch. 14 - Prob. 44TECh. 14 - How many different conformation are possible for...Ch. 14 - Prob. 46TECh. 14 - The temperatures in a fractionating tower at an...Ch. 14 - There are five atoms in the methane molecule, CH4....Ch. 14 - Compared to lighter hydrocarbons, do heavier...Ch. 14 - What do these two structures have in common?Ch. 14 - With four unpaired valence electrons, how can...Ch. 14 - What do the compounds cyclopropane and propene...Ch. 14 - What are the chemical formula for the following...Ch. 14 - Remember that carbon-carbon single bonds can...Ch. 14 - Which of the structures shown in the previous...Ch. 14 - Why are there so many different organic compounds?Ch. 14 - Identify the following functional groups-amide,...Ch. 14 - What must be added to a double bond to transform...Ch. 14 - What do phenols and carboxylic acids have in...Ch. 14 - What is the difference between a ketone and an...Ch. 14 - Prob. 61TECh. 14 - What do alcohols, phenols, and ethers have in...Ch. 14 - Prob. 63TECh. 14 - What is the percent volume of water in 80- proof...Ch. 14 - One of the skin-irritating components of poison...Ch. 14 - Prob. 66TECh. 14 - Prob. 67TECh. 14 - A common inactive ingredient in products such as...Ch. 14 - A common inactive ingredient in products such as...Ch. 14 - The phosphoric acid salt of caffeine has the...Ch. 14 - Prob. 71TECh. 14 - In water, does the following molecule act as an...Ch. 14 - Prob. 73TECh. 14 - The amino acid lysine is shown here. What...Ch. 14 - Why does the carbon of the carbonyl usually have a...Ch. 14 - Prob. 76TECh. 14 - Suggest an explanation for why aspirin has a sour...Ch. 14 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 14 - What products are formed upon the reaction of...Ch. 14 - The disodium salt of ethylenediaminetetraacetic...Ch. 14 - Prob. 81TECh. 14 - Which is better for you: a drug that is a natural...Ch. 14 - Naloxone is a molecule that binds to the opioid...Ch. 14 - What use might there be for Naloxone?Ch. 14 - Rank the following from least ideal to most ideal...Ch. 14 - Why are plastics generally so inexpensive?Ch. 14 - Would you expect polypropylene to be denser or...Ch. 14 - Hydrocarbons release a lot of energy when ignited....Ch. 14 - The polymer styrene-butadiene rubber SBR, shown...Ch. 14 - Citral and camphor are both 10 carbon odoriferous...Ch. 14 - Many of the natural product molecules synthesized...Ch. 14 - The solvent diethyl ether can be mixed with water...Ch. 14 - Alkaloid salts are not very soluble in the organic...Ch. 14 - Go online and look up the total synthesis of the...Ch. 14 - Medicines, such as pain relievers and...Ch. 14 - Why does the melting point of hydrocarbons get...Ch. 14 - Prob. 2RATCh. 14 - Which contains more hydrogen atoms a five-carbon...Ch. 14 - Prob. 4RATCh. 14 - Why might a high-formula-mass alcohol be insoluble...Ch. 14 - Alkaloids salts are not very soluble in the...Ch. 14 - Explain why caprylic acid, CH3(CH2)6COOH,...Ch. 14 - How many oxygen atoms are bonded to the carbon of...Ch. 14 - Prob. 9RATCh. 14 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. If we represent the solar system on a ...
Cosmic Perspective Fundamentals
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Describe the 1H NMR spectrum you would expect for each of the following compounds, indicating the relative posi...
Organic Chemistry (8th Edition)
Modified True/False 9. A giant bacterium that is large enough to be seen without a microscope is Selenomonas.
Microbiology with Diseases by Body System (5th Edition)
In tomato plants, purple leaf color is controlled by a dominant allele A, and green leaf by a recessive allele ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Three slits, each separated from its neighbor by d = 0.06 mm, are illuminated by a coherent light source of wavelength 550 nm. The slits are extremely narrow. A screen is located L = 2.5 m from the slits. The intensity on the centerline is 0.05 W. Consider a location on the screen x = 1.72 cm from the centerline. a) Draw the phasors, according to the phasor model for the addition of harmonic waves, appropriate for this location. b) From the phasor diagram, calculate the intensity of light at this location.arrow_forwardA Jamin interferometer is a device for measuring or for comparing the indices of refraction of gases. A beam of monochromatic light is split into two parts, each of which is directed along the axis of a separate cylindrical tube before being recombined into a single beam that is viewed through a telescope. Suppose we are given the following, • Length of each tube is L = 0.4 m. • λ= 598 nm. Both tubes are initially evacuated, and constructive interference is observed in the center of the field of view. As air is slowly let into one of the tubes, the central field of view changes dark and back to bright a total of 198 times. (a) What is the index of refraction for air? (b) If the fringes can be counted to ±0.25 fringe, where one fringe is equivalent to one complete cycle of intensity variation at the center of the field of view, to what accuracy can the index of refraction of air be determined by this experiment?arrow_forward1. An arrangement of three charges is shown below where q₁ = 1.6 × 10-19 C, q2 = -1.6×10-19 C, and q3 3.2 x 10-19 C. 2 cm Y 93 92 91 X 3 cm (a) Calculate the magnitude and direction of the net force on q₁. (b) Sketch the direction of the forces on qiarrow_forward
- (Figure 1)In each case let w be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w Find the direction of the force exerted on the strut by the pivot in the arrangement (a). Express your answer in degrees. Find the tension Tb in the cable in the arrangement (b). Express your answer in terms of w. Find the magnitude of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in terms of w.arrow_forward(Figure 1)In each case let ww be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w. Find the direction of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in degrees.arrow_forwardA 70.0 cm, uniform, 40.0 N shelf is supported horizontally by two vertical wires attached to the sloping ceiling (Figure 1). A very small 20.0 N tool is placed on the shelf midway between the points where the wires are attached to it. Find the tension in the left-hand wire. Express your answer with the appropriate units.arrow_forward
- Find the total bind Mev. binding energy for 13 Carbon, 6C (atomic mass = 13.0033554)arrow_forwardWhat is the 27 energy absorbed in this endothermic Auclear reaction 2] Al + 'n → 27 Mg + ! H? (The atom mass of "Al is 26.981539u. and that of 11 Mg is 26.984341u) MeVarrow_forwardWhat is the energy released in this nuclear reaction 1 F + "', H-1 O+ He? 19 19 16 (The atomic mass of 1F is 18.998403 u, and that of 20 is 15.9949154) MeV.arrow_forward
- What is the energy released in this B+ nuclear reaction خالد 2½ Al w/ Mg + ie? (The atomic mass of 11 Al is 23.9999394 and that > of 12 Mg is 23.985041 u) MeV.arrow_forwardWhat is the energy released / absorbed in this nuclear reaction 14 N+ & He → » O + ! N? (The atomic mass of 14 N is 14.003074u. 17N+ and that of 10 is 16.9991324). MeVarrow_forwardCan someone help me answer this question thanks.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning


An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY