
Chemistry: Structure and Properties Plus Mastering Chemistry with Pearson eText -- Access Card Package (2nd Edition) (New Chemistry Titles from Niva Tro)
2nd Edition
ISBN: 9780134436524
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 38E
This graph shows a plot of the
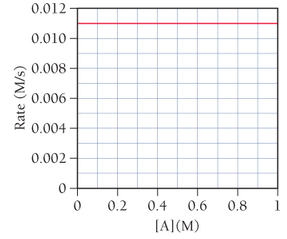
a. What is the order of the reaction with respect to A?
b. Make a rough sketch of a plot of [A] versus time.
c. Write a rate law for the reaction including the value of k.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The
reaction mechanism typically involves a carbocation intermediate.
>
3rd attempt
3343
10
8
Draw arrows to show the reaction between the alkene and hydronium ion.
that
2nd attempt
Feedback
1st attempt
تعمال
Ju See Periodic Table See Hint
F
D
Ju See Periodic Table See Hint
Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product.
4th attempt
Π
Draw the simplified curved arrow mechanism
T
3rd attempt
Feedback
Ju
See Periodic Table See Hint
H
-H
H
-I
H
F
See Periodic Table See Hint
Select the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation
to show how it is converted to the final product.
4th attempt
Part 1 (0.5 point)
Select the correct reagent to accomplish the first step of this reaction.
Choose one:
OA Mg in ethanol (EtOH)
OB. 2 Li in THF
O C. Li in THF
D. Mg in THF
O E Mg in H2O
Part 2 (0.5 point)
Br
Part 1
Bri
Mg
CH
B
CH,
1 Draw intermediate here, but no arrows.
©
TE
See Periodic Table See Hint
See Hint
ין
H
Chapter 14 Solutions
Chemistry: Structure and Properties Plus Mastering Chemistry with Pearson eText -- Access Card Package (2nd Edition) (New Chemistry Titles from Niva Tro)
Ch. 14 - Explain why lizards become sluggish in cold...Ch. 14 - Why are reaction rates important (both practically...Ch. 14 - Using the idea that reactions occur as a result of...Ch. 14 - Using the idea that reactions occur as a result of...Ch. 14 - What units are typically used to express the rate...Ch. 14 - Why is the reaction rate for reactants defined as...Ch. 14 - Explain the difference between the average rate of...Ch. 14 - Consider a simple reaction in which a reactant A...Ch. 14 - How is the order of a reaction generally...Ch. 14 - For a reaction with multiple reactants, how is the...
Ch. 14 - Explain the difference between the rate law for a...Ch. 14 - Write integrated rate laws for zero-order,...Ch. 14 - What does the term half-life mean? Write the...Ch. 14 - How do reaction rates typically depend on...Ch. 14 - Prob. 15ECh. 14 - What is an Arrhenius plot? Explain the...Ch. 14 - Explain the meaning of the orientation factor in...Ch. 14 - Explain the difference between a normal chemical...Ch. 14 - In a reaction mechanism, what is an elementary...Ch. 14 - What are the two requirements for a proposed...Ch. 14 - What is an intermediate within a reaction...Ch. 14 - What is a catalyst? How does a catalyst increase...Ch. 14 - Explain the difference between homogeneous...Ch. 14 - What are the four basic steps involved in...Ch. 14 - What are enzymes? What is the active site of an...Ch. 14 - What is the general two-step mechanism by which...Ch. 14 - Consider the reaction. 2HBr(g)H2(g)+Br2(g) Express...Ch. 14 - Consider the reaction 2N2O(g)2N2(g)+O2(g) Express...Ch. 14 - For the reaction 2A(g)+B(g)3C(g) determine the...Ch. 14 - For the reaction A(g)+12B(g)2C(g) determine the...Ch. 14 - Consider the reaction. Cl2(g)+3F2(g)2ClF3(g)...Ch. 14 - Consider the reaction. 8H2S(g)+4O2(g)8H2O(g)+S8(g)...Ch. 14 - Consider the reaction: C4H8(g)2C2H4(g) The...Ch. 14 - Consider the reaction: NO2(g)NO(g)+12O2(g) The...Ch. 14 - Consider the reaction. H2(g)+Br2(g)2HBr(g) The...Ch. 14 - Consider the reaction. 2H2O2(aq)2H2O(l)+O2(g) The...Ch. 14 - This graph shows a plot of the rate of a reaction...Ch. 14 - This graph shows a plot of the rate of a reaction...Ch. 14 - What are the units of k for each type of reaction?...Ch. 14 - This reaction is first order in N2O5:...Ch. 14 - A reaction in which A, B, and C react to form...Ch. 14 - A reaction in which A, B, and C react to form...Ch. 14 - Consider the tabulated data showing initial rate...Ch. 14 - Consider the tabulated data showing initial rate...Ch. 14 - The tabulated data were collected for this...Ch. 14 - The tabulated data were collected for this...Ch. 14 - Indicate the order of reaction consistent with...Ch. 14 - Indicate the order of reaction consistent with...Ch. 14 - The tabulated data show the concentration of AB...Ch. 14 - The tabulated data show the concentration of N2O5...Ch. 14 - The tabulated data show the concentration of...Ch. 14 - Prob. 52ECh. 14 - This reaction was monitored as a function of time:...Ch. 14 - This reaction was monitored as a function of time:...Ch. 14 - Prob. 55ECh. 14 - Prob. 56ECh. 14 - Prob. 57ECh. 14 - Prob. 58ECh. 14 - The diagram shows the energy of a reaction as the...Ch. 14 - Prob. 60ECh. 14 - Prob. 61ECh. 14 - Prob. 62ECh. 14 - Prob. 63ECh. 14 - The rate constant (k) for a reaction is measured...Ch. 14 - The tabulated data shown here were collected for...Ch. 14 - Prob. 66ECh. 14 - The tabulated data were collected for the...Ch. 14 - Prob. 68ECh. 14 - A reaction has a rate constant of 0.0117/s at...Ch. 14 - A reaction has a rate constant of 0.000122/s at...Ch. 14 - Prob. 71ECh. 14 - Prob. 72ECh. 14 - Prob. 73ECh. 14 - Prob. 74ECh. 14 - Prob. 75ECh. 14 - Prob. 76ECh. 14 - Consider this three-step mechanism for a...Ch. 14 - Prob. 78ECh. 14 - Prob. 79ECh. 14 - Prob. 80ECh. 14 - Suppose that a catalyst lowers the activation...Ch. 14 - The activation barrier for the hydrolysis of...Ch. 14 - The tabulated data were collected for this...Ch. 14 - Prob. 84ECh. 14 - Consider the reaction: A+B+CD The rate law for...Ch. 14 - Consider the reaction: 2O3(g)3O2(g) The rate law...Ch. 14 - At 700 K acetaldehyde decomposes in the gas phase...Ch. 14 - Prob. 88ECh. 14 - Dinitrogen pentoxide decomposes in the gas phase...Ch. 14 - Cyclopropane (C3H6) reacts to form propene (C3H6)...Ch. 14 - Iodine atoms combine to form I2 in liquid hexane...Ch. 14 - Prob. 92ECh. 14 - The reaction AB(aq)A(g)+B(g) is second order in AB...Ch. 14 - The reaction 2H2O2(aq)2H2O(l)+O2(g) is first order...Ch. 14 - Consider this energy diagram: a. How many...Ch. 14 - Consider the reaction in which HCI adds across the...Ch. 14 - The desorption of a single molecular layer of...Ch. 14 - The evaporation of a 120-nm film of n-pentane from...Ch. 14 - Prob. 99ECh. 14 - Prob. 100ECh. 14 - Prob. 101ECh. 14 - Consider the two reactions: O+N2NO+NEa= 315 kJ/mol...Ch. 14 - Anthropologists can estimate the age of a bone or...Ch. 14 - Prob. 104ECh. 14 - Consider the gas-phase reaction: H2(g)+I2(g)2HI(g)...Ch. 14 - Consider the reaction:...Ch. 14 - Prob. 107ECh. 14 - Prob. 108ECh. 14 - A certain substance X decomposes. Fifty percent of...Ch. 14 - Prob. 110ECh. 14 - Prob. 111ECh. 14 - Prob. 112ECh. 14 - Prob. 113ECh. 14 - Prob. 114ECh. 14 - Prob. 115ECh. 14 - Prob. 116ECh. 14 - Phosgene (Cl2CO), a poison gas used in World War...Ch. 14 - The rate of decomposition of N2O3(g) to NO2(g) and...Ch. 14 - At 473 K, for the elementary reaction...Ch. 14 - Prob. 120ECh. 14 - Prob. 121ECh. 14 - A particular reaction, Aproducts has a rate that...Ch. 14 - Prob. 123ECh. 14 - A certain compound, A, reacts to form products...Ch. 14 - Methane (CH4) is a greenhouse gas emitted by...Ch. 14 - This graph shows the concentration of the reactant...Ch. 14 - Prob. 2SAQCh. 14 - Prob. 3SAQCh. 14 - Prob. 4SAQCh. 14 - Prob. 5SAQCh. 14 - Prob. 6SAQCh. 14 - Prob. 7SAQCh. 14 - Prob. 8SAQCh. 14 - The rate constant of a reaction is measured at...Ch. 14 - Prob. 10SAQCh. 14 - The mechanism shown here is proposed for the...Ch. 14 - Prob. 12SAQCh. 14 - These images represent the first-order reaction AB...Ch. 14 - Prob. 14SAQCh. 14 - Prob. 15SAQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY