
Concept explainers
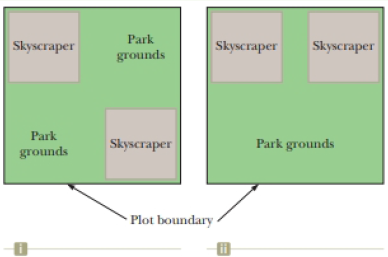
You are working as an expert witness for the owner of a skyscraper complex in a downtown area. The owner is being sued by pedestrians on the streets below his buildings who were injured by falling glass when windows popped outward from the sides of the building. The Bernoulli effect can have important consequences for window’s in such buildings. For example, wind can blow around a skyscraper at remarkably high speed, creating low pressure on the outside surface of the windows. The higher atmospheric pressure in the still air inside the buildings can cause windows to pop out. (a) In your research into the case, you find some overhead views of your client’s project, as shown below. The project includes two tall skyscrapers and some park area on a square plot. Plan (i) (Fig. P14.26(i), page 382) was submitted by the original architects and planners. At the last minute, the owner decided he didn’t want the park grounds to be divided into two areas and submitted Plan (ii) (Fig. P14.26(ii), which is the way the project was built. Explain to your client why Plan (ii) is a much more dangerous situation in terms of windows popping out than Plan (i). (b) Your client is not convinced by your conceptual argument in part (a), so you provide a numerical argument. Suppose a horizontal wind blows with a speed of 11.2 m/s outside a large pane of plate glass with dimensions 4.00 m × 1.50 m. Assume the density of the air to be constant at 1.20 kg/ m3. The air inside the building is at atmospheric pressure. Calculate the total force exerted by air on the windowpane for your client. (c) What If? To further convince your client of the problems with the building design, calculate the total force exerted by air on the windowpane if the wind speed between the buildings is 22.4 m/s, twice as high as in part (b).
Figure P14.26
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
PHYSICS F/ SCI +ENGRS W/ WEBASSIGN ACCES
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
General, Organic, and Biological Chemistry - 4th edition
Microbiology Fundamentals: A Clinical Approach
- 2). How much energy is stored in the 50-μF capacitor when Va - V₁ = 22V? 25 µF b 25 µF 50 µFarrow_forward9). A series RC circuit has a time constant of 1.0 s. The battery has a voltage of 50 V and the maximum current just after closing the switch is 500 mA. The capacitor is initially uncharged. What is the charge on the capacitor 2.0 s after the switch is closed? R 50 V a. 0.43 C b. 0 66 C c. 0.86 C d. 0.99 C Carrow_forward1). Determine the equivalent capacitance of the combination shown when C = 12 pF. +11/20 2C C Carrow_forward
- 3). When a capacitor has a charge of magnitude 80 μC on each plate the potential difference across the plates is 16 V. How much energy is stored in this capacitor when the potential difference across its plates is 42 V? a. 1.0 mJ b. 4.4 mJ c. 3.2 mJ d. 1.4 mJ e. 1.7 mJarrow_forward5). A conductor of radius r, length & and resistivity p has resistance R. It is melted down and formed into a new conductor, also cylindrical, with one fourth the length of the original conductor. The resistance of the new conductor is a. 1 R 161 b. 1 R C. R d. 4R e. 16Rarrow_forward8). Determine the magnitude and sense (direction) of the current in the 10-Q2 resistor when I = 1.8 A. 30 V L 50 V 10 Ω 20 Ω a. 1.6 A right to left b. 1.6 A left to right C. 1.2 A right to left d. 1.2 A left to right e. 1.8 A left to right R PGarrow_forward
- 7). Determine the current in the 10-V emf. 5.0 0 w 10 V 5.0 0 15 V 5.0 Ω a. 2.3 A b. 2.7 A c. 1.3 A d. 0.30 A e. 2.5 Aarrow_forward4). What is the resistance of a wire made of a material with a resistivity of 3.2 is 2.5 m and its diameter is 0.50 mm? a. 0.16 Ω b. 0.10 2 C. c. 1.28 Ω d. 0.41 2 e. 0.81 2 108 m if its lengtharrow_forwardA flat circular coil with 135 turns, a radius of 2.28 x 10-2 m, and a resistance of 0.618 is exposed to an external magnetic field that is directed perpendicular to the plane of the coil. The magnitude of the external magnetic field is changing at a rate of AB/At = 0.615 T/s, thereby inducing a current in the coil. Find the magnitude of the magnetic field at the center of the coil that is produced by the induced current. Numberarrow_forward
- please solve the question attachedarrow_forwardSketch a sine wave depicting 3 seconds of wave activity for a 5 Hz tone. Sketch the resulting complex wave form that results from the combination of the following two waves. Is this wave periodic or aperiodic? USE GRAPH PAPER!arrow_forwardRequired information A bungee jumper leaps from a bridge and undergoes a series of oscillations. Assume g = 9.78 m/s². If a 60.0-kg jumper uses a bungee cord that has an unstretched length of 30.1 m and she jumps from a height of 45.2 m above a river, coming to rest just a few centimeters above the water surface on the first downward descent, what is the period of the oscillations? Assume the bungee cord follows Hooke's law.arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





