
EP BASIC CHEMISTRY-STANDALONE ACCESS
6th Edition
ISBN: 9780134999890
Author: Timberlake
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.4, Problem 21PP
Use the following graph for problems 1.21 and 1.22:Temperature of Tea versus Time for Cooling
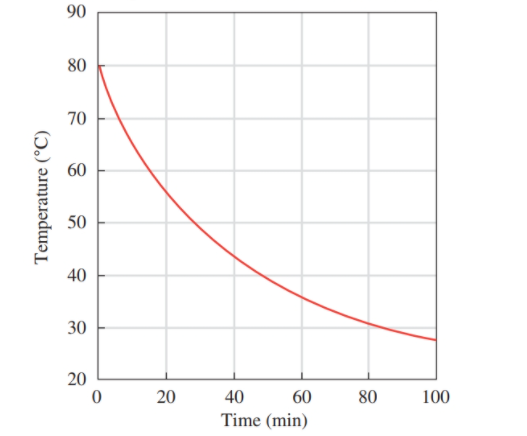
a. What does the title indicate about the graph?
b. What is measured on the vertical axis?
c. What is the range of values on the vertical axis?
d. Does the temperature increase or decrease with an increase in time?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
QUESTION: Find the standard deviation for the 4 different groups
5.298
3.977
223.4
148.7
5.38
4.24
353.7
278.2
5.033
4.044
334.6
268.7
4.706
3.621
305.6
234.4
4.816
3.728
340.0
262.7
4.828
4.496
304.3
283.2
4.993
3.865
244.7
143.6
STDEV =
STDEV =
STDEV =
STDEV =
QUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression'
*The images of the data showing 'coefficients for the standard curve' have been provided
Using the Nernst equation to calculate nonstandard cell voltage
Try Again
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations.
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
2+
2+
Sn²+ Ba(s)
(aq) + Ba (s) Sn (s) + Ba²+ (aq)
→>>
Suppose the cell is prepared with 6.10 M Sn
2+
2+
in one half-cell and 6.62 M Ba
in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
1.71 V
☐ x10
☑
5
0/5
?
00.
18
Ar
Chapter 1 Solutions
EP BASIC CHEMISTRY-STANDALONE ACCESS
Ch. 1.1 - In every chapter. odd-numbered exercises in the...Ch. 1.1 - In every chapter. odd-numbered exercises in the...Ch. 1.1 - Prob. 3PPCh. 1.1 - Prob. 4PPCh. 1.1 - In every chapter. odd-numbered exercises in the...Ch. 1.1 - Prob. 6PPCh. 1.2 - Identify each activity, a to f, as an observation,...Ch. 1.2 - Identify each activity, a to f. as an observation,...Ch. 1.2 - Identify each of the following as an observation,...Ch. 1.2 - Identify each of the following as an observation,...
Ch. 1.3 - Prob. 11PPCh. 1.3 - What are four things that would make it difficult...Ch. 1.3 - Prob. 13PPCh. 1.3 - Prob. 14PPCh. 1.4 - What is the place value for the bold digit? a....Ch. 1.4 - What is the place value for the bold digit? a....Ch. 1.4 - Prob. 17PPCh. 1.4 - Prob. 18PPCh. 1.4 - Prob. 19PPCh. 1.4 - Prob. 20PPCh. 1.4 - Use the following graph for problems 1.21 and...Ch. 1.4 - Use the following graph for problems 1.21 and...Ch. 1.4 - a. A clinic had 25 patients on Friday morning....Ch. 1.4 - a. At a local hospital. 35 babies were born in...Ch. 1.4 - Prob. 25PPCh. 1.4 - Prob. 26PPCh. 1.5 - Prob. 27PPCh. 1.5 - Write each of the following in scientific...Ch. 1.5 - Prob. 29PPCh. 1.5 - Write each of the following as a standard number:...Ch. 1.5 - Prob. 31PPCh. 1.5 - Prob. 32PPCh. 1.5 - Prob. 33PPCh. 1.5 - Prob. 34PPCh. 1 - The chapter sections to review are shown in...Ch. 1 - Prob. 36UTCCh. 1 - Prob. 37UTCCh. 1 - Prob. 38UTCCh. 1 - Prob. 39UTCCh. 1 - Prob. 40UTCCh. 1 - Prob. 41UTCCh. 1 - Prob. 42UTCCh. 1 - Select the correct phrase(s) to complete the...Ch. 1 - Select the correct phrase(s) to complete the...Ch. 1 - Prob. 45APPCh. 1 - Prob. 46APPCh. 1 - Prob. 47APPCh. 1 - Prob. 48APPCh. 1 - Evaluate each of the following: (1.4)...Ch. 1 - Prob. 50APPCh. 1 - A bag of gumdrops contains 16 orange gumdrops, 8...Ch. 1 - Prob. 52APPCh. 1 - Write each of the following in scientific...Ch. 1 - Write each of the following in scientific...Ch. 1 - Prob. 55APPCh. 1 - Write each of the following as a standard number:...Ch. 1 - Prob. 57APPCh. 1 - Prob. 58APPCh. 1 - Prob. 59CPCh. 1 - Prob. 60CPCh. 1 - Prob. 61CPCh. 1 - The following problems are related to the topics...Ch. 1 - The following problems are related to the topics...Ch. 1 - Prob. 64CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forward
- The following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forward
- Briefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forward
- The following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY