
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 14, Problem 164CWP
Consider a 0.60-M solution of HC3H5O3, lactic acid (Ka = 1.4 × 10−4).
a. Which of the following are major species in the solution?
i. HC3H5O3
ii. C3H5O3−
iii. H+
iv. H2O
v. OH−
b. Complete the following ICE table in terms of x, the amount (mol/L) of lactic acid that dissociates to reach equilibrium.
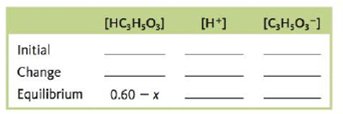
c. What is the equilibrium concentration for C3H3O3−?
d. Calculate the pH of the solution.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Identifying the major species in weak acid or weak base equilibria
Your answer is incorrect.
• Row 2: Your answer is incorrect.
• Row 3: Your answer is incorrect.
• Row 6: Your answer is incorrect.
0/5
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
acids:
HF
0.1 mol of NaOH is added to
1.0 L of a 0.7M HF
solution.
bases:
0.13 mol of HCl is added to
1.0 L of a solution that is
1.0M in both HF and KF.
Exponent
other:
F
acids: HF
bases: F
other:
K
1
0,0,...
?
000
18
Ar
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ
Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions
about this system:
?
rise
Under these conditions, will the pressure of NOCI tend to rise or fall?
x10
fall
Is it possible to reverse this tendency by adding NO?
In other words, if you said the pressure of NOCI will tend to rise, can that
be changed to a tendency to fall by adding NO? Similarly, if you said the
pressure of NOCI will tend to fall, can that be changed to a tendency to
rise by adding NO?
yes
no
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of NO needed to reverse it.
Round your answer to 2 significant digits.
0.035 atm
✓
G
00.
18
Ar
Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area.
HO-
HO-
-0
OH
OH
HO
NG
HO-
HO-
OH
OH
OH
OH
NG
OH
Chapter 14 Solutions
Chemistry
Ch. 14 - Define each of the following: a. Arrhenius acid b....Ch. 14 - Define or illustrate the meaning of the following...Ch. 14 - Define or illustrate the meaning of the following...Ch. 14 - How is acid strength related to the value of Ka?...Ch. 14 - Two strategies are followed when solving for the...Ch. 14 - Two strategies are also followed when solving for...Ch. 14 - Table 13-4 lists the stepwise Ka values for some...Ch. 14 - For conjugate acidbase pairs, how are Ka and Kb...Ch. 14 - What is a salt? List some anions that behave as...Ch. 14 - For oxyacids, how does acid strength depend on a....
Ch. 14 - Consider two beakers of pure water at different...Ch. 14 - Differentiate between the terms strength and...Ch. 14 - Sketch two graphs: (a) percent dissociation for...Ch. 14 - Consider a solution prepared by mixing a weak acid...Ch. 14 - Prob. 5ALQCh. 14 - Consider two separate aqueous solutions: one of a...Ch. 14 - You are asked to calculate the H+ concentration in...Ch. 14 - Consider a solution prepared by mixing a weak acid...Ch. 14 - Consider a solution formed by mixing 100.0 mL of...Ch. 14 - A certain sodium compound is dissolved in water to...Ch. 14 - Acids and bases can be thought of as chemical...Ch. 14 - Consider two solutions of the salts NaX(aq) and...Ch. 14 - What is meant by pH? True or false: A strong acid...Ch. 14 - Why is the pH of water at 25C equal to 7.00?Ch. 14 - Can the pH of a solution be negative? Explain.Ch. 14 - Is the conjugate base of a weak acid a strong...Ch. 14 - Match the following pH values: 1, 2, 5, 6, 6.5, 8,...Ch. 14 - The salt BX, when dissolved in water, produces an...Ch. 14 - Anions containing hydrogen (for example, HCO3 and...Ch. 14 - Which of the following conditions indicate an...Ch. 14 - Which of the following conditions indicate a basic...Ch. 14 - Why is H3O+ the strongest acid and OH the...Ch. 14 - How many significant figures are there in the...Ch. 14 - In terms of orbitals and electron arrangements,...Ch. 14 - Consider the autoionization of liquid ammonia:...Ch. 14 - The following are representations of acidbase...Ch. 14 - Give three example solutions that fit each of the...Ch. 14 - Prob. 28QCh. 14 - Prob. 29QCh. 14 - Which of the following statements is(are) true?...Ch. 14 - Consider the following mathematical expressions....Ch. 14 - Consider a 0.10-M H2CO3 solution and a 0.10-M...Ch. 14 - Of the hydrogen halides, only HF is a weak acid....Ch. 14 - Explain why the following are done, both of which...Ch. 14 - Write balanced equations that describe the...Ch. 14 - Write the dissociation reaction and the...Ch. 14 - For each of the following aqueous reactions,...Ch. 14 - For each of the following aqueous reactions,...Ch. 14 - Classify each of the following as a strong acid or...Ch. 14 - Consider the following illustrations: Which beaker...Ch. 14 - Use Table 13-2 to order the following from the...Ch. 14 - Use Table 13-2 to order the following from the...Ch. 14 - You may need Table 13-2 to answer the following...Ch. 14 - You may need Table 13-2 to answer the following...Ch. 14 - Calculate the [OH] of each of the following...Ch. 14 - Calculate the [H+] of each of the following...Ch. 14 - Values of Kw as a function of temperature are as...Ch. 14 - At 40.C the value of Kw is 2.92 1014. a....Ch. 14 - Calculate the [OH] of each of the following...Ch. 14 - Calculate [H+] and [OH] for each solution at 25C....Ch. 14 - Fill in the missing information in the following...Ch. 14 - Fill in the missing information in the following...Ch. 14 - Prob. 53ECh. 14 - The pOH of a sample of baking soda dissolved in...Ch. 14 - What are the major species present in 0.250 M...Ch. 14 - A solution is prepared by adding 50.0 mL of 0.050...Ch. 14 - Calculate the pH of each of the following...Ch. 14 - Calculate the pH of each of the following...Ch. 14 - Calculate the concentration of an aqueous HI...Ch. 14 - Calculate the concentration of an aqueous HBr...Ch. 14 - How would you prepare 1600 mL of a pH = 1.50...Ch. 14 - A solution is prepared by adding 50.0 mL...Ch. 14 - What are the major species present in 0.250 M...Ch. 14 - What are the major species present in 0.250 M...Ch. 14 - Calculate the concentration of all species present...Ch. 14 - For propanoic acid (HC3H5O2, Ka = 1.3 105),...Ch. 14 - A solution is prepared by dissolving 0.56 g...Ch. 14 - Monochloroacetic acid, HC2H2ClO2, is a skin...Ch. 14 - A typical aspirin tablet contains 325 mg...Ch. 14 - A solution is made by adding 50.0 mL of 0.200 M...Ch. 14 - Calculate the percent dissociation of the acid in...Ch. 14 - Using the Ka values in Table 14.2, calculate the...Ch. 14 - A 0.15-M solution of a weak acid is 3.0%...Ch. 14 - An acid HX is 25% dissociated in water. If the...Ch. 14 - Trichloroacetic acid (CCl3CO2H) is a corrosive...Ch. 14 - The pH of a 0.063-M solution of hypobromous acid...Ch. 14 - A solution of formic acid (HCOOH, Ka = 1.8 104)...Ch. 14 - A typical sample of vinegar has a pH of 3.0....Ch. 14 - One mole of a weak acid HA was dissolved in 2.0 L...Ch. 14 - You have 100.0 g saccharin, a sugar substitute,...Ch. 14 - Write the reaction and the corresponding Kb...Ch. 14 - Write the reaction and the corresponding Kb...Ch. 14 - Prob. 85ECh. 14 - Use Table 14.3 to help order the following acids...Ch. 14 - Use Table 14.3 to help answer the following...Ch. 14 - Use Table 14.3 to help answer the following...Ch. 14 - Calculate the pH of the following solutions. a....Ch. 14 - Calculate [OH], pOH, and pH for each of the...Ch. 14 - What are the major species present in 0.015 M...Ch. 14 - What are the major species present in the...Ch. 14 - What mass of KOH is necessary to prepare 800.0 mL...Ch. 14 - Calculate the concentration of an aqueous Sr(OH)2...Ch. 14 - What are the major species present in a 0.150-M...Ch. 14 - For the reaction of hydrazine (N2H4) in water,...Ch. 14 - Prob. 97ECh. 14 - Calculate the pH of a 0.20-M C2H5NH2 solution (Kb...Ch. 14 - Calculate the pH of a 0.050-M (C2H5)2NH...Ch. 14 - What is the percent ionization in each of the...Ch. 14 - Calculate the percentage of pyridine (C5H5N) that...Ch. 14 - The pH of a 0.016-M aqueous solution of...Ch. 14 - Calculate the mass of HONH2 required to dissolve...Ch. 14 - Write out the stepwise Ka reactions for the...Ch. 14 - Write out the stepwise Ka reactions for citric...Ch. 14 - A typical vitamin C tablet (containing pure...Ch. 14 - Arsenic acid (H3AsO4) is a triprotic acid with Ka1...Ch. 14 - Calculate the pH and [S2] in a 0.10-M H2S...Ch. 14 - Calculate [CO32] in a 0.010-M solution of CO2 in...Ch. 14 - Calculate the pH of a 2.0-M H2SO4 solution.Ch. 14 - Calculate the pH of a 5.0 103-M solution of...Ch. 14 - Arrange the following 0.10 M solutions in order of...Ch. 14 - Arrange the following 0.10 M solutions in order...Ch. 14 - Given that the Ka value for acetic acid is 1.8 ...Ch. 14 - The Kb values for ammonia and methylamine are 1.8 ...Ch. 14 - Determine [OH], [H+], and the pH of each of the...Ch. 14 - Calculate the concentrations of all species...Ch. 14 - Calculate the pH of each of the following...Ch. 14 - Calculate the pH of each of the following...Ch. 14 - Sodium azide (NaN3) is sometimes added to water to...Ch. 14 - Papaverine hydrochloride (abbreviated papH+Cl;...Ch. 14 - An unknown salt is either NaCN, NaC2H3O2, NaF,...Ch. 14 - Consider a solution of an unknown salt having the...Ch. 14 - A 0.050-M solution of the salt NaB has a pH of...Ch. 14 - A 0.20-M sodium chlorobenzoate (NaC7H4ClO2)...Ch. 14 - Prob. 127ECh. 14 - Prob. 128ECh. 14 - Are solutions of the following salts acidic,...Ch. 14 - Are solutions of the following salts acidic,...Ch. 14 - Place the species in each of the following groups...Ch. 14 - Place the species in each of the following groups...Ch. 14 - Place the species in each of the following groups...Ch. 14 - Using your results from Exercise 133, place the...Ch. 14 - Will the following oxides give acidic, basic, or...Ch. 14 - Will the following oxides give acidic, basic, or...Ch. 14 - Identify the Lewis acid and the Lewis base in each...Ch. 14 - Identify the Lewis acid and the Lewis base in each...Ch. 14 - Aluminum hydroxide is an amphoteric substance. It...Ch. 14 - Zinc hydroxide is an amphoteric substance. Write...Ch. 14 - Would you expect Fe3+ or Fe2+ to be the stronger...Ch. 14 - Prob. 142ECh. 14 - A 10.0-mL sample of an HCl solution has a pH of...Ch. 14 - Which of the following represent conjugate...Ch. 14 - A solution is tested for pH and conductivity as...Ch. 14 - The pH of human blood is steady at a value of...Ch. 14 - Hemoglobin (abbreviated Hb) is a protein that is...Ch. 14 - A 0.25-g sample of lime (CaO) is dissolved in...Ch. 14 - At 25C, a saturated solution of benzoic acid (Ka =...Ch. 14 - Calculate the pH of an aqueous solution containing...Ch. 14 - Acrylic acid (CH29CHCO2H) is a precursor for many...Ch. 14 - Classify each of the following as a strong acid,...Ch. 14 - The following illustration displays the relative...Ch. 14 - Quinine (C20H24N2O2) is the most important...Ch. 14 - Codeine (C18H21NO3) is a derivative of morphine...Ch. 14 - A codeine-containing cough syrup lists codeine...Ch. 14 - Prob. 157AECh. 14 - Rank the following 0.10 M solutions in order of...Ch. 14 - Is an aqueous solution of NaHSO4 acidic, basic, or...Ch. 14 - Calculate the value for the equilibrium constant...Ch. 14 - Prob. 161AECh. 14 - For solutions of the same concentration, as acid...Ch. 14 - Prob. 163CWPCh. 14 - Consider a 0.60-M solution of HC3H5O3, lactic acid...Ch. 14 - Consider a 0.67-M solution of C2H5NH2 (Kb = 5.6 ...Ch. 14 - Rank the following 0.10 M solutions in order of...Ch. 14 - Consider 0.25 M solutions of the following salts:...Ch. 14 - Calculate the pH of the following solutions: a....Ch. 14 - Consider 0.10 M solutions of the following...Ch. 14 - The pH of 1.0 108 M hydrochloric acid is not...Ch. 14 - Calculate the pH of a 1.0 107-M solution of NaOH...Ch. 14 - Calculate [OH] in a 3.0 107-M solution of Ca(OH)2.Ch. 14 - Consider 50.0 mL of a solution of weak acid HA (Ka...Ch. 14 - Prob. 174CPCh. 14 - Calculate the pH of a 0.200-M solution of C5H5NHF....Ch. 14 - Determine the pH of a 0.50-M solution of NH4OCl....Ch. 14 - Calculate [OH] in a solution obtained by adding...Ch. 14 - What mass of NaOH(s) must be added to 1.0 L of...Ch. 14 - Consider 1000. mL of a 1.00 104-M solution of a...Ch. 14 - Calculate the mass of sodium hydroxide that must...Ch. 14 - Consider the species PO43, HPO42, and H2PO4. Each...Ch. 14 - Calculate the pH of a 0.10-M solution of sodium...Ch. 14 - Will 0.10 M solutions of the following salts be...Ch. 14 - a. The principal equilibrium in a solution of...Ch. 14 - A 0.100-g sample of the weak acid HA (molar mass =...Ch. 14 - A sample containing 0.0500 mole of Fe2(SO4)3 is...Ch. 14 - A 2.14 g sample of sodium hypoiodite is dissolved...Ch. 14 - Isocyanic acid (HNCO) can be prepared by heating...Ch. 14 - A certain acid, HA, has a vapor density of 5.11...Ch. 14 - An aqueous solution contains a mixture of 0.0500 M...Ch. 14 - For the following, mix equal volumes of one...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- € + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward
- 03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forward
- Calculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forward
- Select the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forwardSelect the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY