
Concept explainers
(a)
Interpretation:
The number of nodal planes perpendicular to the bonding axes of each of the three
Concept introduction:
Overlap of atomic orbitals (AOs) can be thought of as wave interference. It can be constructive or destructive. Constructive interference increases the electron density between the two nuclei (an antinode) and results in a molecular orbital (MO) of lower energy. The phases of the overlapping orbitals are the same in this case. Destructive interference decreases the electron density between the two nuclei and results in an MO of higher energy. The phases of such AOs are opposite. Since the electron density between the two nuclei decreases, there is a node (or a nodal plane) between the two atoms.
Answer to Problem 14.27P
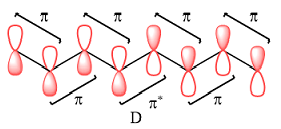
The number of nodal planes is one for MO A, six for MO B, and none for MO C.
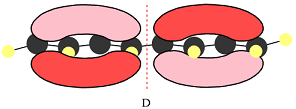
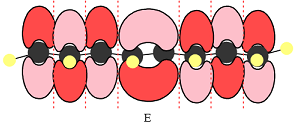
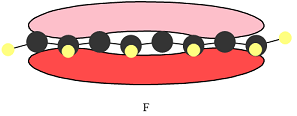
The order of increasing energy of the three MOs is
Explanation of Solution
The phases of the wave function of the two AOs on either side of a nodal plane are opposite each other. This results in destructive interference reducing the electron density to zero at the nodal plane.
Therefore, the nodal planes in the three MOs are:



The energy of the MO increases with the number of nodes. Therefore, the order of increasing energy of the three orbitals is
When atomic orbitals with different phases overlap, a node (zero electron density) is formed at the center, increasing the energy of the MO.
(b)
Interpretation:
The p orbital AO contributions on each carbon atom that would give rise to each MO are to be drawn.
Concept introduction:
The p orbitals that contribute to
Answer to Problem 14.27P
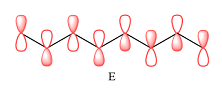
The p orbitals that contribute to each of the three MOs are
Explanation of Solution
In case of D, there are two four-center MOs. Therefore, p orbitals that contribute to it must have the same phase. The second MO has the opposite phase. The p AOs that contribute to this MO will all have the same phase, but it will be opposite to that of the first group.
Therefore, the p orbitals that contribute to MOs shown in A are

E shows a total of five MOs. The first three from left are present in individual atoms. The fourth one is a two-center MO, followed again by three individual MOs. Only the AOs of C4 and C5 must have matching phases. All other adjacent AOs will have mismatched phases.
Therefore, the p orbitals contributing to the MOs as shown in D are

In case of F, a single MO covers all eight carbon atoms. Therefore, the contributing AOs must all have the same phase.

The p orbitals contributing to each MO are determined on the basis of the phases and the presence of an adjacent nodal plane.
(c)
Interpretation:
Each internuclear region is to be identified as having a bonding or an antibonding type of interaction.
Concept introduction:
A bonding interaction arises when the phases of the interacting AOs are the same. This increases the electron density between the two nuclei and lowers the energy of the MO. An antibonding interaction arises when the phases of the interacting AOs are different. This decreases the electron density between the two nuclei to zero at the center and increases the energy of the MO.
Answer to Problem 14.27P
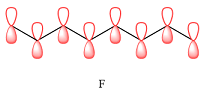
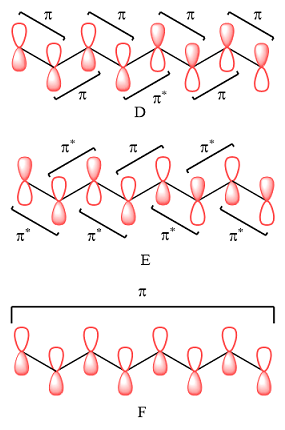
The bonding (
Explanation of Solution
A bonding interaction requires p orbitals of the same phase on adjacent atoms. An antibonding interaction requires the interacting p orbitals to be of opposite phases. An antibonding interaction leads to a nodal plane between the two atoms.
Therefore, in D, there are six bonding interactions. There is only one antibonding interaction between C4 and C5 orbitals.
In case of E, there is only one bonding interaction between C4 and C5 orbitals. All other interactions are antibonding interactions.
In case of F, all are bonding interactions.
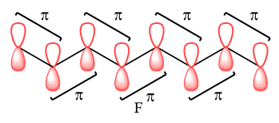
The type of interaction is determined on the basis of the phases of interacting AOs.
(d)
Interpretation:
Whether each MO is overall bonding, nonbonding, or antibonding is to be determined on the basis of the answer to part (c).
Concept introduction:
If the number of bonding interactions are more than the number of antibonding interactions, the MO is overall bonding. If the number of antibonding interactions is more than that of bonding interactions, the MO is overall antibonding. If the numbers are equal or there are no interactions, the MO is overall nonbonding.
Answer to Problem 14.27P
The MO shown in D is overall bonding. MO E is overall antibonding. MO F is overall antibonding. MO C is overall bonding.
Explanation of Solution
There are six bonding and only one antibonding interaction in this case. Therefore, the MO shown in D is overall bonding.
In the case of E, there is only one bonding interaction and six antibonding interactions. Therefore, MO E is overall antibonding.
All interactions in MO C are bonding interactions. Therefore, this MO is overall bonding.
The overall character of a multicenter MO is determined by the numbers of bonding and antibonding interactions.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry: Principles And Mechanisms
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
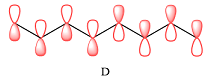
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
- Identify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





