
KLEIN'S ORGANIC CHEMISTRY
3rd Edition
ISBN: 9781119423126
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 13, Problem 65IP
Interpretation Introduction
Interpretation: The mechanism for the transformation of compound
Concept Introduction:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid. This process is stereospecific.
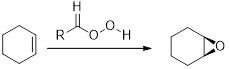
LiAH4 (Lithium aluminium hydride):
Lithium aluminium hydride is used as a reducing agent. Lithium aluminium hydride is reduced the

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The process to determine the configuration, starts by placing the lowest priority
substituent toward the back.
If the substituents pointing forward decrease in priority in a clockwise order, the
configuration is S. If the substituents decrease in priority in a counterclockwise order,
the configuration is R.
True
False
In the drawing area below, create a hemiacetal with 1 hydroxyl group, 1 methoxy group, and a total of 3 carbon atoms.
Click and drag to start drawing a
structure.
Explanation
Check
Х
PO
18
Ar
B
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Predict the product of the reaction below (3 pts).
hydrazine
Ph
H₂NNH2
KOH
Write the mechanism for the above reaction using curved arrows to show electron movements.
show all intermediates in the process (7 pts).
Chapter 13 Solutions
KLEIN'S ORGANIC CHEMISTRY
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - PRACTICE the skill
Show reagents that you could...Ch. 13.5 -
APPLY the skill
Compound 2 was made as a...Ch. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 16PTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 18PTSCh. 13.10 -
The sequence below shows an enantioselective...Ch. 13.11 - Prob. 20CCCh. 13.11 - Prob. 21CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 22PTSCh. 13.12 - Prob. 23ATSCh. 13.12 - Prob. 7LTSCh. 13.12 - Prob. 24PTSCh. 13.12 - Prob. 25ATSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Methylmagnesium bromide reacts rapidly with...Ch. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46IPCh. 13 - Prob. 47IPCh. 13 - Prob. 48IPCh. 13 - Prob. 49IPCh. 13 - Prob. 50IPCh. 13 - Prob. 51IPCh. 13 - Prob. 52IPCh. 13 - Prob. 53IPCh. 13 - Prob. 54IPCh. 13 - Prob. 55IPCh. 13 - Prob. 56IPCh. 13 - Prob. 57IPCh. 13 - Prob. 58IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 67IPCh. 13 - Prob. 68IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - The following procedure13 is part of a synthetic...Ch. 13 - Prob. 72CPCh. 13 - Prob. 73CPCh. 13 - Prob. 74CPCh. 13 - Prob. 75CP
Knowledge Booster
Similar questions
- ↓ Feedback (8/10) Draw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 2 attempts remaining N H3O+ 0 × Select to Draw + V Retryarrow_forward2. Calculate the branching ratio of the reaction of the methyl peroxy radical with either HO, NO 298K) (note: rate constant can be found in the tropospheric chemistry ppt CH,O,+NO-HCHO+HO, + NO, CH₂O+HO, CH₂00H +0₂ when the concentration of hydroperoxyl radical is DH01-1.5 x 10 molecules and the nitrogen oxide maxing ratio of 10 ppb when the concentration of hydroperoxyl radicalis [H0] +1.5x10 molecules cm" and the nitrogen oxide mixing ratio of 30 p Under which condition do you expect more formaldehyde to be produced and whyarrow_forwardIndicate the product of the reaction of benzene with 1-chloro-2,2-dimethylpropane in the presence of AlCl3.arrow_forward
- In what position will N-(4-methylphenyl)acetamide be nitrated and what will the compound be called.arrow_forwardDATA: Standard Concentration (caffeine) mg/L Absorbance Reading 10 0.322 20 0.697 40 1.535 60 2.520 80 3.100arrow_forwardIn what position will p-Toluidine be nitrated and what will the compound be called.arrow_forward
- In what position will 4-methylbenzonitrile be nitrated and what will the compound be called.arrow_forwardIn what position will benzenesulfonic acid be nitrated?arrow_forwardIf compound A reacts with an excess of methyl iodide and then heated with aqueous Ag₂O, indicate only the major products obtained. Draw their formulas. A Harrow_forward
- Explanation Check 1:01AM Done 110 Functional Groups Identifying and drawing hemiacetals and acetals In the drawing area below, create a hemiacetal with 1 ethoxy group, 1 propoxy group, and a total of 9 carbon atoms. Click and drag to start drawing a structure. ✓ $ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Sarrow_forwardWrite the systematic name of each organic molecule: CI structure CI CI Explanation CI ठ CI Check B ☐ 188 F1 80 name F2 F3 F4 F5 F6 60 F7 2arrow_forwardWrite the systematic name of each organic molecule: structure i HO OH Explanation Check name ☐ ☐arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY