
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059223
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 39TC
Rank these reaction profiles in order of increasing reaction speed:
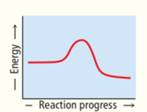
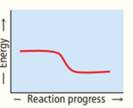
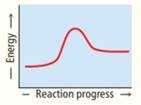
(a) (b) (c)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
!
Required information
Two chloride ions and two sodium ions are in water, the "effective charge" on the chloride ions (CI¯) is −2.00 × 10-21 C
and that of the sodium ions (Na+) is +2.00 x 10-21 C. (The effective charge is a way to account for the partial shielding due
to nearby water molecules.) Assume that all four ions are coplanar.
CT
Na+
Na+
30.0°
45.0%
с
сг
L.
where a = 0.300 nm, b = 0.710 nm, and c = 0.620 nm.
What is the direction of electric force on the chloride ion in the lower right-hand corner in the diagram? Enter the angle in degrees
where positive indicates above the negative x-axis and negative indicates below the positive x-axis.
A pendulum has a 0.4-m-long cord and is given a tangential velocity of 0.2 m/s toward the
vertical from a position 0 = 0.3 rad.
Part A
Determine the equation which describes the angular motion.
Express your answer in terms of the variable t. Express coefficients in radians to three significant figures.
ΜΕ ΑΣΦ
vec
(t)=0.3 cos (4.95t) + 0.101 sin (4.95t)
Submit Previous Answers Request Answer
× Incorrect; Try Again; 6 attempts remaining
Part A
■Review
The uniform 150-lb stone (rectangular block) is being turned over on its side by pulling the
vertical cable slowly upward until the stone begins to tip.
(Figure 1)
If it then falls freely (T = 0) from an essentially balanced at-rest position, determine the speed at which the corner A strikes the pad at B. The stone does not slip at its corner C as it falls. Suppose that height of the stone is
L = 1.2 ft.
Express your answer to three significant figures and include the appropriate units.
?
ft
VA 10.76
S
Submit Previous Answers Request Answer
× Incorrect; Try Again; 6 attempts remaining
Chapter 13 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 13 - Prob. 1RCCCh. 13 - Prob. 2RCCCh. 13 - Prob. 3RCCCh. 13 - If it takes 436 kilojoules to break a bond, how...Ch. 13 - What is released by an exothermic reaction?Ch. 13 - What is absorbed by an endothermic reaction?Ch. 13 - Prob. 7RCCCh. 13 - What generally happens to the rate of a chemical...Ch. 13 - Which reactant molecules are the first to pass...Ch. 13 - How is an acid different from a base?
Ch. 13 - When an acid is dissolved in water, what ion does...Ch. 13 - Prob. 12RCCCh. 13 - Are there many hydronium ions in neutral water?Ch. 13 - What is true about the relative concentrations of...Ch. 13 - What does the pH of a solution indicate?Ch. 13 - Prob. 16RCCCh. 13 - What elements have the greatest tendency to behave...Ch. 13 - What happens to a reducing agent as it reduces?Ch. 13 - What metal coats a galvanized nail?Ch. 13 - What is iron forced to accept during cathodic...Ch. 13 - What happens to the polarity of oxygen atoms as...Ch. 13 - What catalyst is effective in the destruction of...Ch. 13 - Prob. 23TISCh. 13 - What net effect does a chemical reaction have on a...Ch. 13 - What is the product of the reaction between carbon...Ch. 13 - Prob. 26TISCh. 13 - Prob. 27TISCh. 13 - A material that tends to lose electrons is put...Ch. 13 - What is the primary difference between a battery...Ch. 13 - Prob. 30TISCh. 13 - Rank these reaction profiles in order of...Ch. 13 - Rank the covalent bonds in order of increasing...Ch. 13 - Review the concept of electronegativity in Section...Ch. 13 - Review the concept of electronegativity in Section...Ch. 13 - Rank these molecules from least oxidized to most...Ch. 13 - Prob. 44TSCh. 13 - Prob. 45TSCh. 13 - When the hydronium ion concentration of a solution...Ch. 13 - When the pH of a solution is 1, the concentration...Ch. 13 - Show that the pH of a solution is 0.301 when its...Ch. 13 - Show that the hydroxide ion concentration of a...Ch. 13 - How can 50g of wood burn to produce more than 50g...Ch. 13 - Balance these equations: a Fe(s)+O2(g)Fe2O3(s) b...Ch. 13 - Balance these equations: a Fe(s)+S(s)Fe2S3(s) b...Ch. 13 - Prob. 53TECh. 13 - Use the following illustration to answer exercises...Ch. 13 - Use the following illustration to answer exercises...Ch. 13 - What changes during a chemical reaction?Ch. 13 - Prob. 58TECh. 13 - Is photosynthesis an exothermic or endothermic...Ch. 13 - Why does blowing into a campfire make the fire...Ch. 13 - In the laboratory, endothermic reactions are...Ch. 13 - Prob. 62TECh. 13 - Why does a glowing splint of wood burn only slowly...Ch. 13 - Prob. 64TECh. 13 - Chew a salt-free soda cracker for a few minutes...Ch. 13 - Prob. 66TECh. 13 - Does the ozone pollution from automobiles help...Ch. 13 - Chlorine is put into the atmosphere by volcanoes...Ch. 13 - Prob. 69TECh. 13 - Prob. 70TECh. 13 - An acid and a base react to form salt, which...Ch. 13 - Identify the acid or base behavior of each...Ch. 13 - Prob. 73TECh. 13 - Prob. 74TECh. 13 - The main component of bleach is sodium...Ch. 13 - Prob. 76TECh. 13 - Prob. 77TECh. 13 - Within a neutral solution of supercritical water...Ch. 13 - What is the concentration of hydronium ions in a...Ch. 13 - Can an acidic solution be made less acidic by...Ch. 13 - How does burning fossil fuels lower the pH of the...Ch. 13 - Bubbling carbon dioxide into water causes the pH...Ch. 13 - Pour vinegar onto beach sand from the Caribbean,...Ch. 13 - What happens to the pH of soda water as it loses...Ch. 13 - Prob. 85TECh. 13 - Prob. 86TECh. 13 - Why is the chlorine atom such a strong oxidizing...Ch. 13 - Prob. 88TECh. 13 - What element behaves as the oxidizing agent in the...Ch. 13 - Hydrogen sulfide, H2S, burns in the presence of...Ch. 13 - Unsaturated fatty acids, such as C12H22O2, react...Ch. 13 - The type of iron that the human body needs for...Ch. 13 - Why is lithium a preferred metal for the making of...Ch. 13 - Chemical equations must be balanced not only in...Ch. 13 - Study question 94 before attempting to balance...Ch. 13 - How does turning on the radio while you are...Ch. 13 - What are some key advantages that a fuel-cell...Ch. 13 - Do our bodies gradually oxidize or reduce the food...Ch. 13 - Pennies manufactured after 1982 are made of zinc...Ch. 13 - Water is 88.88 oxygen by mass. Oxygen is exactly...Ch. 13 - Why is the air over an open flame always moist?Ch. 13 - Upon ingestion, grain alcohol, C2H6O, is...Ch. 13 - Prob. 103TDICh. 13 - Can industries be trusted to self-regulate the...Ch. 13 - In the centralized model for generating...Ch. 13 - Prob. 1RATCh. 13 - Prob. 2RATCh. 13 - How much energy, in kilojoules, is released or...Ch. 13 - The yeast in bread dough feeds on sugar to produce...Ch. 13 - What role do CFCs play in the catalytic...Ch. 13 - What is the relationship between the hydroxide ion...Ch. 13 - When the hydronium ion concentration equals 1 mole...Ch. 13 - Prob. 8RATCh. 13 - Why does a battery that has thick zinc walls last...Ch. 13 - What element is oxidized in this equation and what...
Additional Science Textbook Solutions
Find more solutions based on key concepts
WHAT IF? Most prairies experience regular fires, typically every few years. If these disturbances were relative...
Campbell Biology in Focus (2nd Edition)
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
53. This reaction was monitored as a function of time:
A plot of In[A] versus time yields a straight ...
Chemistry: Structure and Properties (2nd Edition)
In rabbits, chocolate-colored fur (w+) is dominant to white fur (w), straight fur (c+) is dominant to curly fur...
Genetic Analysis: An Integrated Approach (3rd Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Which compound is more easily decarboxylated?
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Consider the circuit shown in the figure. The battery has emf ε = 69 volts and negligible internal resistance. The inductance is L = 0.4 H and the resistances are R 1 = 12 Ω and R 2 = 9.0 Ω. Initially the switch S is open and no currents flow. Then the switch is closed. After leaving the switch closed for a very long time, it is opened again. Just after it is opened, what is the current in R 1?arrow_forwardA capacitor with a capacitance of C = 5.95×10−5 F is charged by connecting it to a 12.5 −V battery. The capacitor is then disconnected from the battery and connected across an inductor with an inductance of L = 1.55 H . At the time 2.35×10−2 s after the connection to the inductor is made, what is the current in the inductor? At that time, how much electrical energy is stored in the inductor?arrow_forwardCan someone help me with this question. Thanks.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY