
(a)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid. This process is stereospecific.
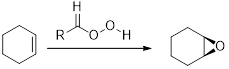
Reaction of Grignard reagent with epoxide will form alcohols. Grignard reagent will add to the less substituted carbon. The C-Mg bond in the Grignard reagent will act as the nucleophile.
(b)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Alkoxymercuration-demercuration: the process where alcohols can be prepared from alkene, results in the markovnikov’s addition of H & OH groups across the double bond of alkene.
NaBH4 (Sodium borohydride):
Sodium borohydride is used as a reducing agent.
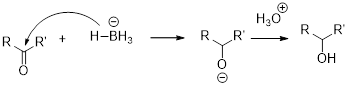
(c)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Epoxides from Peroxy acids:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid. This process is stereospecific.
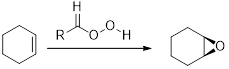
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions
(d)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Reaction of metal with alcohol:
Metals can react with alcohol to produce alkoxide ion. For an example, ethanol can be react with sodium to produce hydrogen gas and sodium ethoxide.
(e)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Reaction of metal with alcohol:
Metals can react with alcohol to produce alkoxide ion. For an example, ethanol can be react with sodium to produce hydrogen gas and sodium ethoxide.
Epoxides from Peroxy acids:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid. This process is stereospecific.
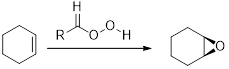
Acid-catalyzed ring-opening of epoxide: The epoxide ring is protonated and the nucleophile attack depends on the electronic or steric effect (nature of epoxide).
Regiochemistry: when the epoxide is unsymmetrical, the nucleophile attack at the more substituted position of the protonated epoxide ring.
Stereochemistry: when the nucleophile attack takes place at chiral center, an inversion of configuration is obtained.
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions
(f)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Reaction of Grignard reagent with epoxide will form alcohols. Grignard reagent will add to the less substituted carbon. The C-Mg bond in the Grignard reagent will act as the nucleophile.
The reactions of Epoxides with strong nucleophiles requires a strong driving force that helps in the removal of ring strain associated with the three-membered ring of an Epoxide. These ring opening reactions also occur under acidic conditions.
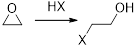
The mechanism of acid-catalyzed ring opening of an Epoxide occurs in two steps.
In first step, the protonation of Epoxide occurs.
In second step, the attack of nucleophile occurs in the protonated Epoxide by SN2 process.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
ORGANIC CHEMISTRYPKGDRL+MLCRL MDL
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- At 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardPredict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





