
Interpretation: The experimental procedure to test the hypothesis that solute blocking of solvent vaporization is not the reason that solutions have lower vapor pressures than the pure solvents is to be determined.
Concept introduction: According to Raoult’s law, the vapor pressure of a solution is equal to the product of the mole fraction and the vapor pressure of the pure solvent.
The expression of Raoult’s law is,
Answer to Problem 1DE
Solution: The interaction between solute and solvent and the number of solute particles are responsible for the lower vapor pressures of the solution than the pure solvents.
Explanation of Solution
According to Raoult’s law, the vapor pressure of a solution is equal to the product of the mole fraction and the vapor pressure of the pure solvent.
The expression of Raoult’s law is,
When a solute is added to a solution, the vapor pressure of the solution decreases because on addition of solute, the gap between the solvent molecules gets filled. Hence, on the surface of solution, the number of solvent molecule is less as compared to the pure solvent. This indicates that the number of solvent molecule enter into the gas phase is less and due to this there is a decrease in vapor pressure.
Using the Raoult’s law, the lowering of vapor pressure can be explained.
Assume two sealed containers, one is for pure solvent and other is for solution. The equilibrium stage arises when the number of molecules striking on the surface of the molecule becomes equal to the number of molecules going to the gas phase. On addition of solute, half of the surface of the solution is occupied by the solute and half of the surface is occupied by solvent molecules.
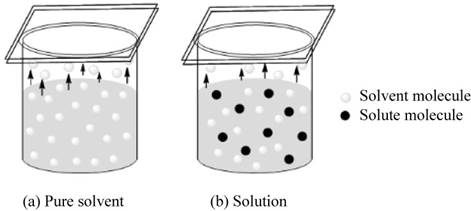
Figure 1
The vapor pressure of the solution depends upon the number of solvent molecule present on the surface of the solution. As the number of solute molecule increases, the number of solvent molecule present on the surface of solution decreases, now lesser number of solvent molecules is present to go in gas phase. Hence, the vapor pressure of the solution decreases.
The attraction between molecules of solvent with solute also plays an important role in lowering the vapor pressure. If there is a strong attraction between the solute and solvent, than the solvent molecule try to remain in the solution rather than escaping from it. Due to this, lowering of vapor pressure occurs because now there are less number of solvent molecule in vapor phase.
Another important factor is that, number of molecules present in the solution is not important rather than this number of solute particles present in the solution is important.
More the number of solute particles, low will be the vapor pressure.
The interaction between solute and solvent and the number of solute particles are responsible for the lower vapor pressures of the solution than the pure solvents.
Want to see more full solutions like this?
Chapter 13 Solutions
EP CHEMISTRY:CENTRAL SCI.-MOD.MASTERING
- Correct each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO—CH,—C—CH,—OH X 5 2 2 2 HO–CH,—CH,—C—CH,—OH Explanation Check Center Accessi ©2025 on 5 Carrow_forwardMake the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).arrow_forwardH CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forward
- Homework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward
- 1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forward
- In the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





