
Concept explainers
Interpretation:
The molecular weight of each explosive in this chemical connection should be determined along with identify which explosive do the nitro groups contribute the largest percentage of molecular weight.
Concept Introduction:
The following explosives are present in the chemical connections.
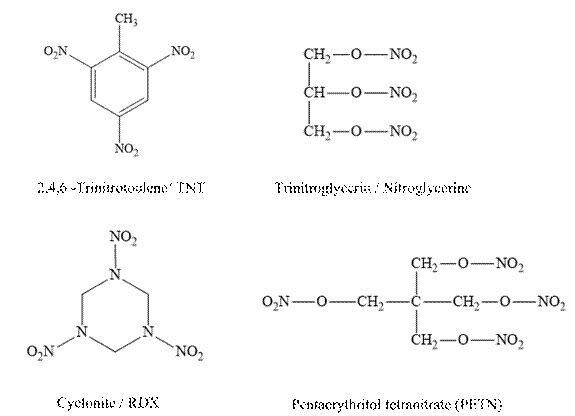
Answer to Problem 13.41P
Molecular weight of TNT is
Molecular weight of nitroglycerine is.
Molecular weight of cyclonite is.
Molecular weight of pentaerythritol is
Therefore, cyclonite has high percentage contribution of nitro group in their molecular weight.
Explanation of Solution
Molecular weight of TNT (
Atomic weight of total number of carbon atoms =
Atomic weight of total number of hydrogen atoms =
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =
Molecular weight =
Therefore, molecular weight of TNT is
Percentage composition of
TNT has three
Weight of.
Therefore, the percentage contribution of.
Percentage contribution of nitrogen in TNT:
TNT has three nitrogens.
Therefore, the percentage contribution of nitrogen in TNT is.
Molecular weight of nitroglycerine (
Atomic weight of total number of carbon atoms =
Atomic weight of total number of hydrogen atoms =
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =
Molecular weight =
Therefore, molecular weight of nitroglycerine is
Percentage composition of
Nitroglycerine has three
Weight of.
Therefore, the percentage contribution of.
Percentage contribution of nitrogen in nitroglycerine:
Nitroglycerine has three nitrogens.
Therefore, the percentage contribution of nitrogen in nitroglycerine is
Molecular weight of cyclonite (
Atomic weight of total number of carbon atoms =.
Atomic weight of total number of hydrogen atoms =.
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =
Molecular weight =
Therefore, molecular weight of cyclonite is
Percentage composition of
Cyclonite has three
Weight of
Therefore, the percentage contribution of.
Percentage contribution of nitrogen in cyclonite:
Cyclonite has six nitrogens.
Therefore, the percentage contribution of nitrogen in cyclonite is.
Molecular weight of pentaerythritol (
Atomic weight of total number of carbon atoms =
Atomic weight of total number of hydrogen atoms =
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =.
Molecular weight =
Therefore, molecular weight of pentaerythritol is
Percentage composition of
Pentaerythritol has four
Weight of
Therefore, the percentage contribution of
Percentage contribution of nitrogen in pentaerythritol
Pentaerythritol has four nitrogens.
Therefore, the percentage contribution of nitrogen in pentaerythritol is
Molecular weight of TNT is
Molecular weight of nitroglycerine is
Molecular weight of cyclonite is
Molecular weight of pentaerythritol is
Therefore, cyclonite has high percentage contribution of nitro group in their molecular weight.
Want to see more full solutions like this?
Chapter 13 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
- Complete the following equations please hand written pleasearrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 3Cu²+ (aq) +2Al(s) → 3 Cu(s)+2A1³* (aq) 2+ Suppose the cell is prepared with 5.29 M Cu in one half-cell and 2.49 M A1³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 μ ☑ 00. 18 Ar Иarrow_forwardPlease help me solve this homework problemarrow_forward
- Please help me answer this homework questionarrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3+ H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq) 0 kJ x10 Х ? olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3) NH with a 0.3000M solution of HClO4. The pK₁ of dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it. 2 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 000 18 Ar 1 Barrow_forward
- Using the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq) + 2+ 2+ 3+ Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ x10 μ Х 5 ? 000 日。arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq) kJ - ☐ x10 x10 olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
