
(a)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
(![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
The starting ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
Explanation of Solution
The given synthesis scheme is:
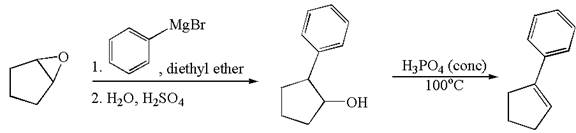
In the given synthetic route, the reactant having functional group epoxide is converted to an alcohol named ![]() which is further converted to an
which is further converted to an ![]() on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is
on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is ![]() which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and
which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and ![]() is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
The starting epoxide reacts with phenylmagnesium bromide in the solvent diethyl ether followed by aqueous acid, to form ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
The given synthesis scheme is converted to word form by identifying the names of reactants, reagents, and products.
(b)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to products by reacting with the reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane, to produce phenylpropanone. Then, add the molecular bromine in the presence of acetic acid to form ![]() which further reacts with sodium acetate to yield the final product.
which further reacts with sodium acetate to yield the final product.
Explanation of Solution
The given synthesis scheme is:
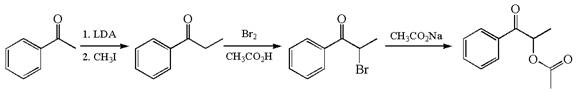
The given synthetic route is of three steps. In the first step, the reactant having functional group ![]() alpha bromo ketone named
alpha bromo ketone named ![]() , which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
, which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane to produce phenylpropanone. Add the molecular bromine in presence of acetic acid to phenylpropanone to form ![]() which further reacts with sodium acetate to yield THE final product.
which further reacts with sodium acetate to yield THE final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
(c)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , add phosphorous tribromide to it to produce
, add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
Explanation of Solution
The given synthesis scheme is:
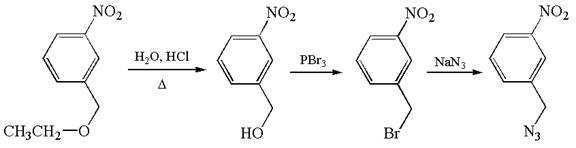
The given synthetic route is of three steps. The nitro functional group remains as it is throughout the reaction sequence, and so, is not considered. In the first step, ether functional group is converted to an alcohol. In the second step, ![]() group of alcohol is replaced by bromine, which in the third step is replaced by
group of alcohol is replaced by bromine, which in the third step is replaced by ![]() . In the first step, the reagent used is
. In the first step, the reagent used is ![]() that represents acidic condition where
that represents acidic condition where ![]() is the symbol used for heat. In the second step, the reagent is
is the symbol used for heat. In the second step, the reagent is ![]() , phosphorous tribromide, and in the third step, the reagent used is sodium azide,
, phosphorous tribromide, and in the third step, the reagent used is sodium azide, ![]() . Thus, the word form of the above synthetic scheme can be written as follows:
. Thus, the word form of the above synthetic scheme can be written as follows:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , and add phosphorous tribromide to it to produce
, and add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
Want to see more full solutions like this?
Chapter 13 Solutions
Get Ready for Organic Chemistry
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
- Identify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward
- 6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forwardWhat is the mechanism for this?arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
