
To find:
a) Write the rate law for the given reaction and the value of the rate constant at
b) Calculate the activation energy of the reaction from given data.
Answer to Problem 13.142QA
Solution:
a) The rate law for the given reaction is
b) The activation energy of the reaction is
Explanation of Solution
1) Concept:
We can write the rate law for the reaction as
We need to find the values of x, y to write complete rate law and then find the rate constant k of the reaction.
2) Given:
We are given the table for the values of initial concentrations and initial rates of
| Experiment | Initial Rate |
||
3) Calculations:
a) We need to first find the order of reaction with respect to
Using the general equation of rate law,
We can write rate equations for all the experiments as
Now by taking the ratio of equation (1) and (2), we can find value of x.
Now, to find value of y, we have to take ratio of equation (2) and equation (3) and put
Thus, we can write the rate law equation as
1. Using this rate law and the values of concentrations and initial rates, we can find the rate constant,
i) So from experiment 1, rate law is
ii) From experiment 2, rate law is
iii) From experiment 3, rate law is
The average value of rate constant
b) To find the activation energy, we have to plot the graph of
We have given table for the values of rate constants at different temperatures.
The plot of
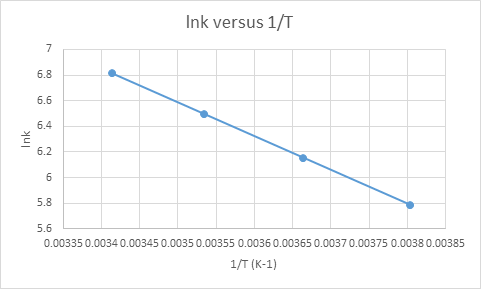
The activation energy would be
Conclusion:
a) The rate law for the given reaction is
b) The activation energy of the reaction is
Want to see more full solutions like this?
Chapter 13 Solutions
SMARTWORKS FOR CHEMISTRY: ATOMS FOCUSED
- Can I please get help with this?arrow_forwardUse the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





