
Concept explainers
Methane is to be adiabatically and reversibly compressed from 50 psia and 100°F to 500 psia. Calculate the specific work required for this compression treating the methane as an ideal gas with variable specific heats and using the departure charts.
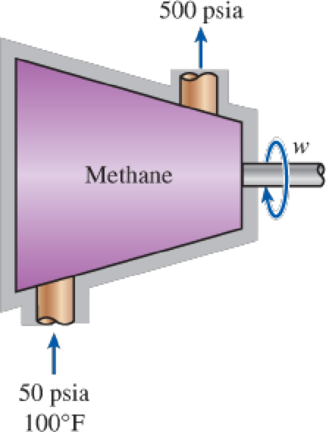
FIGURE P12–96E
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats and using departure charts.
Answer to Problem 96RP
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
The work input of the compressor per unit mass of the methane using departure charts is
Explanation of Solution
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
The general empirical correlation is
Write the formula for enthalpy change in molar basis at ideal gas state
Here, specific heat capacity at constant pressure is
Write the formula for work input to the compressor
Here, molar mass of methane is
Write the formula for entropy change in molar basis at ideal gas state
Here, universal gas constant is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Write the formula for change in enthalpy
Here, change in enthalpy of ideal gas is
Refer Table A-1E, “Molar mass, gas constant, and critical-point properties”.
The critical temperature and critical pressure of the methane is as follows.
Refer table A-1E, “Molar mass, gas constant and critical properties table”.
The molar mass
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
Obtain the empirical constants as follows.
Refer Table A-2E (a), “Ideal-gas specific heats of various common gases”.
The gas constant
The specific heat at constant pressure
The universal gas constant
Conclusion:
Convert the inlet temperature from degree Fahrenheit to Rankine.
It is given that the compression process in reversible adiabatic process. Hence, the change in entropy during the process is zero.
Substitute
By using Engineering Equation Solver (EES) or online calculator to solve the Equation (XI) and obtain the value of
Substitute
Substitute
Thus, the work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Consider the final temperature
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Substitute
Here, the work input of the compressor is equal to the enthalpy difference.
Thus, the work input of the compressor per unit mass of the methane using departure charts is
Want to see more full solutions like this?
Chapter 12 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- I tried this problem a couple of times and don't know where I'm going wrong can you help me out pleasearrow_forwardy(0)=1, Using Laplace transforms solve the following differential equations : 11) y"-4y+4y=0, 12) y+2y+2y=0, y(0)=2.1, y'(0) = 3.9 y'(0)=-3. 13) y+7y+12y=21e", y(0)=3.5, y'(0)=-10. 14) +9y=10e. y(0)=0, y'(0) = 0. 15) y+3y+2.25y=91³ +64, y(0)=1, y'(0) = 31.5 16) -6y+5y= 29 cos(21), y(0)=3.2, y'(0)=6.2 17) "+2y+2y=0, y(0)=0, y'(0)=1. 18) +2y+17y=0, y(0)=0, y'(0)=12. 19) y-4y+5y=0, y(0)=1, y'(0) = 2. 20) 9y-6y+y=0, y(0)=3, y'(0)=1. 21) -2y+10y=0, y(0)=3, y'(0)=3.arrow_forward4. Consider the rectangulan 2535 Let 16 a and section discussed 977b + class. in ie make a M thin" rectangle, Can you you show that Q = Go {a² = x² } . Imax = 2 Ga ты J =arrow_forward
- 1. Consider a circular shaft in torsion that of radius r=b has a key way as shown, circle of radius a Let us try the solution x₁ (5,0) = k (6² = r²) (1- 2 awso 1.1 Does this solve the problem for the stres rer 1,2 Solve for is and 23.arrow_forward3. - a For an elliptical cross that the tangent to section resultant shear can you s stress is show ellipse with the same 24 i ratio of eccentricity, in passes through to point alb that in question, it + Parrow_forward2. Consider the rod with an elliptical that strain 4 a Cross secton considered in class, Integrate the was displacement displacements, relations to obtain thearrow_forward
- Please answer Oxygen at 300 kPa and 90°C flowing at an average velocity of 3 m/s is expanded in an adiabatic nozzle. What is the maximum velocity of the oxygen at the outlet of this nozzle when the outlet pressure is 60 kPa? Use the table containing the ideal gas specific heats of various common gases. The maximum velocity of the oxygen at the outlet of this nozzle is 532.5 Numeric ResponseEdit Unavailable. 532.5 incorrect.m/s.arrow_forwardA container filled with 70 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer between the water and the air in the room. Assume the room is at the sea level, well sealed, and heavily insulated. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Determine the amount of heat transfer between the water and the air in the room. The amount of heat transfer between the water and the air in the room is kJ.arrow_forwardA strain gauge rosette that is attached to the surface of a stressed component gives 3 readings (ɛa = A, b = B, &c = C). If the strain gauge rosette is of the D° type (indicating the angle between each of the gauges), construct a Mohr's Strain Circle overleaf. You should assume that gauge A is aligned along the x-axis. Using the Mohr's Strain Circle calculate the: (i) principal strains (ε1, 2)? (ii) principal angles (1, 2)? You should measure these anticlockwise from the y-axis. (iii) maximum shear strain in the plane (ymax)?arrow_forward
- Q1. If the yield stress (σy) of a material is 375MPa, determine whether yield is predicted for the stresses acting on both the elements shown below using: (a) Tresca Criterion (b) Von Mises Criterion P Element A R S Element B Note: your values for P (vertical load on Element A) should be negative (i.e. corresponding to a compressive vertical load).arrow_forwardQ. After a puncture a driver is attempting to remove a wheel nut by applying a force of P KN to one end of a wheel brace as shown in Fig. 1. In cross-section the brace is a hollow steel tube (see section aa) of internal diameter r mm and external diameter q mm. wheel nut n Position S P m r q Section aa Fig, 1 (a) Calculate (i) the twisting moment, (ii) the bending moment, and (iii) the shear force in the brace at position S due to the applied load P. (b) Calculate (i) the shear stress due to twisting, and (ii) the bending stress at position S. Note that the shear force will not produce any shear stress at S. (c) Calculate the maximum shearing stress in the brace at position S using the Maximum Shear Stress Criterion. 2 Mechanics of Materials 2 Tutorials Portfolio: Exercise 5 (d) If the maximum permissible shear stress in the steel is 200 MPa, determine the maximum torque that can be applied by the brace without the risk of failure at S.arrow_forwardCalculate the first 5 Fourier series coefficients (A0-4 and B1-5 ) for the estimated R wave.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





