
Concept explainers
(a)
Interpretation:
Whether the statement “A single bond between carbon and nitrogen is polar” is true or false is to be stated.
Concept introduction:
The polar bond is a type of covalent bond. It is a bond in which the electrons are shared unequally between the two atoms because of which one atom becomes partially negative and the other becomes partially positive.
Answer to Problem 42E
The statement “A single bond between carbon and nitrogen is polar” is true.
Explanation of Solution
In the molecule of
Therefore, the
The given statement is true.
(b)
Interpretation:
Whether the statement “A bond between phosphorus and sulfur will be less polar than a bond between phosphorus and chlorine” is true or false is to be stated.
Concept introduction:
The polar bond is a type of covalent bond. It is a bond in which the electrons are shared unequally between the two atoms because of which one atom becomes partially negative and the other becomes partially positive.
Answer to Problem 42E
The statement “A bond between phosphorus and sulfur will be less polar than a bond between phosphorus and chlorine” is true.
Explanation of Solution
The strength of the polarity between bonds is based on the electronegativity of the bond. The difference in electronegativity between the atoms of phosphorus and chlorine is
On the other hand, the difference in electronegativity between the atoms of phosphorus and sulfur is
Therefore,
The given statement is true.
(c)
Interpretation:
Whether the statement “The electronegativity of calcium is less than the electronegativity of aluminum” is true or false is to be stated.
Concept introduction:
Electronegativity is defined as the tendency of an atom to attract electrons towards it. Polarized bonds are a result of the electronegativity difference between bonding atoms. If the electronegativity difference is
Answer to Problem 42E
The statement “The electronegativity of calcium is less than the electronegativity of aluminum” is true.
Explanation of Solution
Electronegativity is the capability of an atom to attract the shared pair of electron towards itself.
On moving left to right in a period, the electronegativity increases.
Since, calcium is a
Therefore, aluminum is more electronegative than calcium.
The given statement is true.
(d)
Interpretation:
Whether the statement “Strontium
Concept introduction:
The ions or atoms which possess the same electron configuration are known as the isoelectronic species. The meaning of isoelectronic species is the species which contains the equal charge. The isoelectronic species shows the similar chemical properties.
Answer to Problem 42E
The statement “Strontium
Explanation of Solution
The isoelectronic species are those in which the number of electrons are same.
The electronic configuration of
The electronic configuration of
Since the number of electrons in
Therefore,
The given statement is true.
(e)
Interpretation:
Whether the statement “The monoatomic ion formed by selenium
Concept introduction:
The ions or atoms which possess the same electron configuration are known as the isoelectronic species. The meaning of isoelectronic species is the species which contains the equal charge. The isoelectronic species shows the similar chemical properties.
Answer to Problem 42E
The statement “The monoatomic ion formed by selenium
Explanation of Solution
The isoelectronic species are those in which the number of electrons are same.
The
Therefore, the statement “The monoatomic ion formed by selenium
The given statement is true.
(f)
Interpretation:
Whether the statement “Most elements in Group
Concept introduction:
The monoatomic ion is one that is formed by a single atom. In a montoatomic ion, the number of protons and electrons are different. The difference in the number of protons and electrons gives the charge to the atom. When the number of proton is more then the atom attains a positive charge. When the number of electrons is more then the atom acquires a negative charge.
Answer to Problem 42E
The statement “Most elements in Group
Explanation of Solution
The elements of group
So, the statement “Most elements in Group
The given statement is true.
(g)
Interpretation:
Whether the statement “Multiple bonds can form only between atoms of the same element” is true or false is to be stated.
Concept introduction:
The forces that holds various constituents such as atoms, ions or elements together and forms a chemical compound is known as chemical bonding.
The
Answer to Problem 42E
The statement “Multiple bonds can form only between atoms of the same element” is false.
Explanation of Solution
The bonds between the different elements can be more than one. The example to this is the molecule of
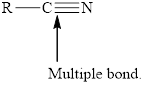
Figure 1
The Figure 1 shows
Therefore, the statement “Multiple bonds can form only between atoms of the same element” is false.
The given statement is false.
(h)
Interpretation:
Whether the statement “If an atom is triple-bonded to another atom, it may still form a bond with one additional atom” is true or false is to be stated.
Concept introduction:
The forces that hold various constituents such as atoms, ions or elements together and forms a chemical compound is known as chemical bonding.
The chemical bonds hold together the atoms in the molecules.
Answer to Problem 42E
The statement “If an atom is triple-bonded to another atom, it may still form a bond with one additional atom” is true.
Explanation of Solution
In order to complete the octet, the triple bonded atoms can form bond with the other atom. The example to this is the molecule of
![]()
Figure. 2
In Figure 2, the carbon atom is triply bonded with nitrogen atom but still its octet is not complete. Therefore, to complete its octet, it forms a bond with the hydrogen atom.
So, the statement “If an atom is triple-bonded to another atom, it may still form a bond with one additional atom” is true.
The given statement is true.
(i)
Interpretation:
Whether the statement “An atom that conforms to the octet rule can bond to no more than three other atoms if one bond is a double bond” is true or false is to be stated.
Concept introduction:
The forces that hold various constituents such as atoms, ions or elements together and forms a chemical compound is known as chemical bonding.
The chemical bonds hold together the atoms in the molecules.
Answer to Problem 42E
The statement “An atom that conforms to the octet rule can bond to no more than three other atoms if one bond is a double bond” is true.
Explanation of Solution
When an atom attains eight electrons in its outermost shell then it is known to have a complete octet.
When the atom is bonded with the other atom with a double bond then it can form single bonds with only two other atoms and therefore, its octet will be completed.
So, the statement “An atom that conforms to the octet rule can bond to no more than three other atoms if one bond is a double bond” is true.
The given statement is true.
(j)
Interpretation:
Whether the statement “Electrons are localized between atoms in a metal” is true or false is to be stated.
Concept introduction:
The substance that has high electrical conductivity and that readily donates electrons in order to attain a positive charge is defined as a metal. Most of the metals react with oxygen to produce oxides.
Answer to Problem 42E
The statement “Electrons are localized between atoms in a metal” is false.
Explanation of Solution
The electrons are present inside the atoms and not between the atoms. So, the localization of electrons between the atom is not possible.
Therefore, the statement “Electrons are localized between atoms in a metal” is false.
The given statement is false.
(k)
Interpretation:
Whether the statement “Alloys are pure substances” is true or false is to be stated.
Concept introduction:
The fusion of two or more metals that chemically combines together is known as an alloy. The fusion of metal with a non-metal is also possible. An alloy is a homogenous mixture, so, it retains the properties of metal even when it is made up of a non-metal or metalloid.
Answer to Problem 42E
The statement “Alloys are pure substances” is false.
Explanation of Solution
An alloy is formed by a mixture of two or more metals or one metal and other non-metal. So, an alloy can not be pure, rather it is a mixture.
Therefore, the statement “Alloys are pure substances” is false.
The given statement is false.
Want to see more full solutions like this?
Chapter 12 Solutions
Introductory Chemistry: An Active Learning Approach
- A student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forwardCalculate the density of 21.12 g of an object that displaces 0.0250 L of water.arrow_forward
- Draw the expected reactant R28. Cu(II) CO₂Mearrow_forwardPpplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward
- 1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forwardReaction Fill-ins Part 2! Predict the product(s) OR starting material of the following reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put your answers in the indicated boxes d. d. ง HCIarrow_forward
- A cylinder contains 12 L of water vapour at 150˚C and 5 atm. The temperature of the water vapour is raised to 175˚C, and the volume of the cylinder is reduced to 8.5 L. What is the final pressure of the gas in atmospheres? assume that the gas is idealarrow_forwardOn the next page is an LC separation of the parabens found in baby wash. Parabens are suspected in a link to breast cancer therefore an accurate way to quantitate them is desired. a. In the chromatogram, estimate k' for ethyl paraben. Clearly indicate what values you used for all the terms in your calculation. b. Is this a "good" value for a capacity factor? Explain. c. What is the resolution between n-Propyl paraben and n-Butyl paraben? Again, indicate clearly what values you used in your calculation. MAU | Methyl paraben 40 20 0 -2 Ethyl paraben n-Propyl paraben n-Butyl paraben App ID 22925 6 8 minarrow_forwardd. In Figure 4, each stationary phase shows some negative correlation between plate count and retention factor. In other words, as k' increases, N decreases. Explain this relationship between k' and N. Plate Count (N) 4000 3500 2500 2000 1500 1000 Figure 4. Column efficiency (N) vs retention factor (k') for 22 nonionizable solutes on FMS (red), PGC (black), and COZ (green). 3000 Eluent compositions (acetonitrile/water, A/W) were adjusted to obtain k' less than 15, which was achieved for most solutes as follows: FMS (30/70 A/W), PGC (60/40), COZ (80/20). Slightly different compositions were used for the most highly retained solutes. All columns were 50 mm × 4.6 mm id and packed with 5 um particles, except for COZ, which was packed with 3 um particles. All other chromatographic conditions were constant: column length 5 cm, column j.§. 4.6 mm, flow rate 2 mL/min, column temperature 40 °C, and injection volume 0.5 μL Log(k'x/K'ethylbenzene) FMS 1.5 1.0 0.5 0.0 ཐྭ ཋ ཤྩ བྷྲ ; 500 0 5 10…arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





