
(a)
The shape of the molecule
N 2 O
(a)
Answer to Problem 32E

The molecule
Explanation of Solution
Given Info: Refer to Table 12.6 in the textbook to predict the shape of the molecule.
Explanation:
The shape of the molecule

The central atom has two surrounding regions.
The polarity of a molecule depends on the electronegativity of atoms in that molecule and the shape of the molecule. A molecule which has one polar bond is polar.
A molecule which has all nonpolar bonds is nonpolar.
The symmetric molecule will be nonpolar because the polarity of bonds is canceled out. Thus an asymmetric molecule is polar because the polarity of bonds is not canceled.
Therefore, the asymmetric molecule
Conclusion:
Therefore, the shape of the molecule

The molecule
(b)
The shape of the molecule
SO 4 2 −
(b)
Answer to Problem 32E
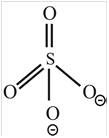
The molecule
Explanation of Solution
Given Info: Refer to Table 12.6 in the textbook to predict the shape of the molecule.
Explanation:
The shape of the molecule

The central atom has four surrounding regions.
The polarity of a molecule depends on the electronegativity of atoms in that molecule and the shape of the molecule. A molecule which has one polar bond is polar.
A molecule which has all nonpolar bonds is nonpolar.
The symmetric molecule will be nonpolar because the polarity of bonds is canceled out. Thus an asymmetric molecule is polar because the polarity of bonds is not canceled.
Therefore, the symmetric molecule
Conclusion:
Therefore, the shape of the molecule

The molecule
(c)
The shape of the molecule
GaH 3
(c)
Answer to Problem 32E
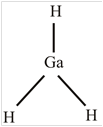
The molecule
Explanation of Solution
Given Info: Refer to Table 12.6 in the textbook to predict the shape of the molecule.
Explanation:
The shape of the molecule

The central atom has three surrounding regions.
The polarity of a molecule depends on the electronegativity of atoms in that molecule and the shape of the molecule. A molecule which has one polar bond is polar.
A molecule which has all nonpolar bonds is nonpolar.
The symmetric molecule will be nonpolar because the polarity of bonds is canceled out. Thus an asymmetric molecule is polar because the polarity of bonds is not canceled.
Therefore, the symmetric molecule
Conclusion:
The shape of the molecule

The molecule
(d)
The shape of the molecule
NH 2 Cl
(d)
Answer to Problem 32E
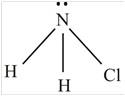
The molecule
Explanation of Solution
Given Info: Refer to Table 12.6 in the textbook to predict the shape of the molecule.
Explanation:
The shape of the molecule

The central atom has four surrounding regions.
The polarity of a molecule depends on the electronegativity of atoms in that molecule and the shape of the molecule. A molecule which has one polar bond is polar.
A molecule which has all nonpolar bonds is nonpolar.
The symmetric molecule will be nonpolar because the polarity of bonds is canceled out. Thus an asymmetric molecule is polar because the polarity of bonds is not canceled.
Therefore, the asymmetric molecule
Conclusion:
Therefore, the shape of the molecule

The molecule
Want to see more full solutions like this?
Chapter 12 Solutions
INTRO TO PHYSICAL SCIENCE W/MINDTAP
- Hi! I need help with these calculations for part i and part k for a physics Diffraction Lab. We used a slit width 0.4 mm to measure our pattern.arrow_forwardExamine the data and % error values in Data Table 3 where the angular displacement of the simple pendulum decreased but the mass of the pendulum bob and the length of the pendulum remained constant. Describe whether or not your data shows that the period of the pendulum depends on the angular displacement of the pendulum bob, to within a reasonable percent error.arrow_forwardIn addition to the anyalysis of the graph, show mathematically that the slope of that line is 2π/√g . Using the slope of your line calculate the value of g and compare it to 9.8.arrow_forward
- An object is placed 24.1 cm to the left of a diverging lens (f = -6.51 cm). A concave mirror (f= 14.8 cm) is placed 30.2 cm to the right of the lens to form an image of the first image formed by the lens. Find the final image distance, measured relative to the mirror. (b) Is the final image real or virtual? (c) Is the final image upright or inverted with respect to the original object?arrow_forwardConcept Simulation 26.4 provides the option of exploring the ray diagram that applies to this problem. The distance between an object and its image formed by a diverging lens is 5.90 cm. The focal length of the lens is -2.60 cm. Find (a) the image distance and (b) the object distance.arrow_forwardPls help ASAParrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax



