
Interpretation:
For each transformation, the reducing agent used, out of
Concept introduction:
Lithium aluminium hydride and sodium borohydride are reducing agents.
Answer to Problem 1PP
Solution:
(a)
(b)
(c)
(d)
(e)
(a)
Explanation of Solution
The given transformation is as follows:
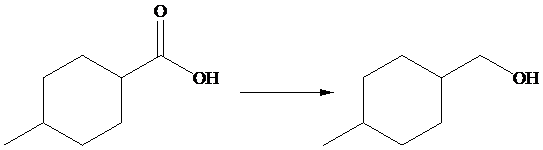
In the above transformation,
Hence,
(b)
The given transformation is as follows;
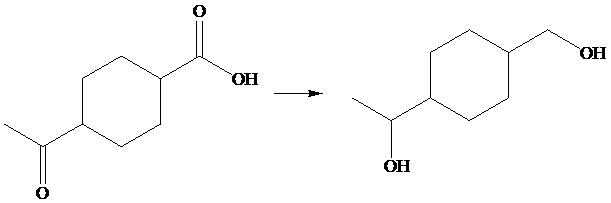
In the above transformation, carboxylic acid and ketone are converted into their corresponding alcohols. This transformation is done by using
Hence,
(c)
The given transformation is as follows:
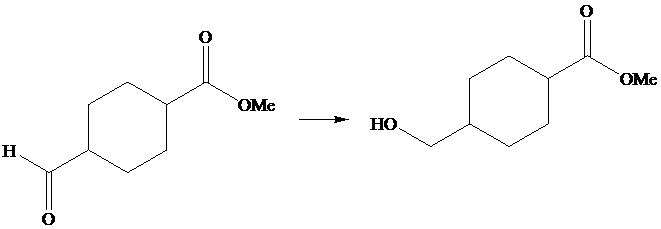
In the above transformation, aldehyde is converted into its corresponding alcohol, but there is no effect on the ester group. This transformation is done by using
Hence,
(d)
The given transformation is as follows:
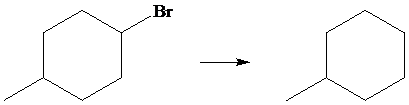
In the above transformation, the bromine atom is replaced by the hydrogen atom. This transformation is done by using
Hence,
(e)
The given transformation is as follows:
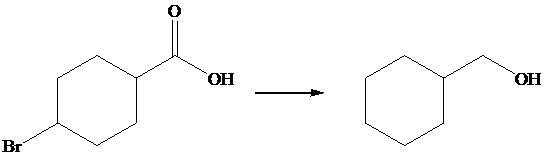
In the above transformation, the bromine atom is replaced by the hydrogen atom, and carboxylic acid is converted into its corresponding alcohol. This transformation is done by using
Hence,
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
- (a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forwardPlease helparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





