
Chemical/Bio Engineering
Figure P12.12 depicts a chemical exchange process consisting of a series of reactors in which a gas fl owing from left to right is passed over a liquid fl owing from right to left. The transfer of a chemical from the gas into the liquid occurs at a rate that is proportional to the difference between the gas and liquid concentrations in each reactor. At steady state, a mass balance for the first reactor can be written for the gas as
and for the liquid as
Where
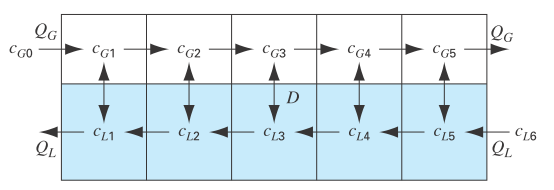
FIGURE P12.12
To calculate: The flow rate of the flow through every pipe if the flow equation through he reactors is provided by following system of equations:
For gas it is provided as:
And for liquid it is provided as:
Answer to Problem 12P
Solution: The concentration of liquid and gases in the reactor is:
Explanation of Solution
Given Information:
The system of reactors is provided as follows:
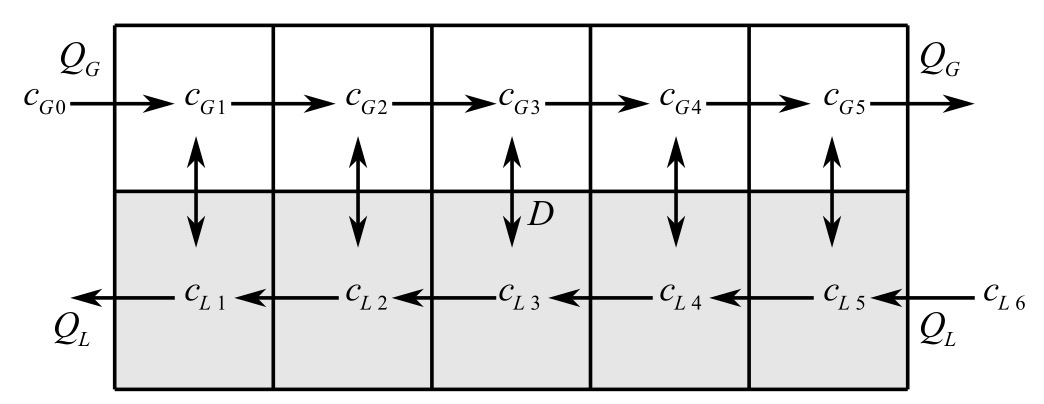
Flow rate and the concentration are provided as:
Formula used:
Write system of linear equations in matrix form.
And,
The term
Calculation:
Consider the figure below,

Here,
For reactor 1, the system is in steady state. Therefore, themass balance equation for the gas reactor 1.
Substitute
For reactor 2, the system is in steady state. Therefore, the mass balance for the gas reactor 2 is,
Substitute
For reactor 3, the system is in steady state, therefore, the mass balance equation for the gas reactor 3 is,
Substitute
For reactor 4, the system is in steady state. Therefore, the mass balance for the gas reactor 4 is,
Substitute
For reactor 5, the system is in steady state. Therefore, the mass balance for the gas reactor 5 is,
Substitute
For reactor 1, the system is in steady state. Therefore, the mass balance for the liquid reactor 1 is,
Substitute
For reactor 2, the system is in steady state. Therefore, the mass balance for the liquid reactor 2 is,
Substitute
For reactor 3, the system is in steady state. Therefore, the mass balance for the liquid reactor 3 is,
Substitute
For reactor 4, the system is in steady state. Therefore, the mass balance for the liquid reactor 4 is,
Substitute
For reactor 5, the system is in steady state. Therefore, the mass balance for the liquid reactor 5 is,
Substitute
Now, recollect all the linear equations for mass balance equation of gases and liquids.
There are too many linear equations which is complex to solving manually. So, write the equation in the form ofmatrices that is augmented form as shown below:
With the help of the linear system of equations provided above, the coefficient matrix A is,
With the help of the linear system of equations provided above, the column matrix X is:
And With the help of the linear system of equations provided above, the column matrix D is,
Write the system of equation in the augmented form.,
Solve the matrix
MATLAB is used to perform the calculation, type the following code into MATLAB cmd.
Once you press eneter, the resut is obtained as follows:
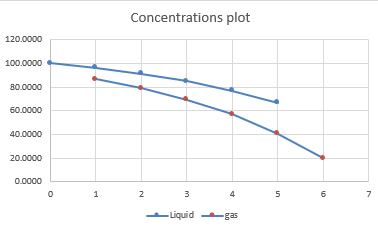
Hence, the values of concentrations passing through the reactors is shown in the below table:
The plot of concentration of liquid and gas is provided as:
Want to see more full solutions like this?
Chapter 12 Solutions
EBK NUMERICAL METHODS FOR ENGINEERS
Additional Engineering Textbook Solutions
Calculus: Early Transcendentals (2nd Edition)
Pathways To Math Literacy (looseleaf)
College Algebra Essentials (5th Edition)
Precalculus
Thinking Mathematically (6th Edition)
Elementary and Intermediate Algebra: Concepts and Applications (7th Edition)
- To generate leads for new business, Gustin Investment Services offers free financial planning seminars at major hotels in Southwest Florida. Gustin conducts seminars for groups of 25 individuals. Each seminar costs Gustin $3,700, and the average first-year commission for each new account opened is $5,200. Gustin estimates that for each individual attending the seminar, there is a 0.01 probability that individual will open a new account. (a) Determine the equation for computing Gustin's profit per seminar, given values of the relevant parameters. Profit = (3,700 x 5,200) - New Accounts Opened Profit = 5,200 - (New Accounts Opened x 3,700) Profit = (New Accounts Opened x 3,700) - 5,200 Profit = New Accounts Opened - (5,200 × 3,700) Profit = (New Accounts Opened x 5,200) - 3,700 (b) What type of random variable is the number of new accounts opened? Hint: Review Appendix 12.1 for descriptions of various types of probability distributions. continuous integer uniform normal discrete uniform…arrow_forwardStrassel Investors buys real estate, develops it, and resells it for a profit. A new property is available, and Bud Strassel, the president and owner of Strassel Investors, believes if he purchases and develops this property, it can then be sold for $158,000. The current property owner has asked for bids and stated that the property will be sold for the highest bid in excess of $100,000. Two competitors will be submitting bids for the property. Strassel does not know what the competitors will bid, but he assumes for planning purposes that the amount bid by each competitor will be uniformly distributed between $100,000 and $148,000. (a) What is the estimate of the probability Strassel will be able to obtain the property using a bid of $128,000? (Use at least 5,000 trials. Round your answer three decimal places.) (b) How much does Strassel need to bid to be assured of obtaining the property? $128,000 $138,000 $148,000 (c) Use the simulation model to compute the profit for each trial of…arrow_forwardGrear Tire Company has produced a new tire with an estimated mean lifetime mileage of 34,500 miles. Management also believes that the standard deviation is 4,500 miles and that tire mileage is normally distributed. To promote the new tire, Grear has offered to refund a portion of the purchase price if the tire fails to reach 30,000 miles before the tire needs to be replaced. Specifically, for tires with a lifetime below 30,000 miles, Grear will refund a customer $1 per 100 miles short of 30,000. Construct a simulation model to answer the following questions. (Use at least 1,000 trials.) (a) For each tire sold, what is the average cost of the promotion (in $)? (Round your answer to two decimal places.) (b) What is the probability that Grear will refund more than $25 for a tire? (Round your answer to three decimal places.)arrow_forward
- Major League Baseball's World Series is a maximum of seven games, with the winner being the first team to win four games. Assume that the Atlanta Braves and the Minnesota Twins are playing in the World Series and that the first two games are to be played in Atlanta, the next three games at the Twins' ballpark, and the last two games, if necessary, back in Atlanta. Taking into account the projected starting pitchers for each game and the home field advantage, suppose the probabilities of Atlanta winning each game are as follows. Game 1 2 3 4 5 6 7 Probability of Win 0.61 0.54 0.47 0.46 0.47 0.56 0.49 Construct a simulation model in which whether Atlanta wins or loses each game is a random variable. Use the model to answer the following questions. (Use at least 1,000 trials.) (a) What is the average number of games played regardless of winner? (Round your answer to one decimal place.) games (b) What is the probability that the Atlanta Braves win the World Series? (Round your answer to…arrow_forward1 Brinkley 2 A B с D E F G H I J 3 Parameters 4 Selling Price 5 Procurement Cost 6 Labor Cost 7 Transportation Cost 8 9 Procurement Cost 10 Lower End of Interval Upper End of Interval Cost Probability 11 $10.00 12 $11.00 0.25 0.45 13 $12.00 0.3 14 15 Labor Cost 16 Lower End of Interval Upper End of Interval Cost Probability 17 $20.00 0.1 18 $22.00 0.25 19 $24.00 0.35 20 $25.00 0.3 21 22 Transportation Cost 23 Lower End of Interval Upper End of Interval Cost Probability 24 25 $3.00 $5.00 0.75 0.25 26 27 Model 28 Profit Per Unit 29 30 Simulation Trial Procurement Cost Labor Cost Transportation Cost Profit Per Unit Summary Statistics 31 1 Mean Profit Per Unit #DIV/0! 32 2 P(Profit <$5) #DIV/0! 83 3 34 4 35 5 36 6 37 7 38 8 39 9 40 10arrow_forwardModel File Available: Download WeddingIMS.xlsx The wedding date for a couple is quickly approaching, and the wedding planner must provide the caterer an estimate of how many people will attend the reception so that the appropriate quantity of food is prepared for the buffet. The following table contains information on the number of RSVP guests for the 145 invitations. Unfortunately, the number of guests does not always correspond to the number of RSVPed guests. Based on her experience, the wedding planner knows it is extremely rare for guests to attend a wedding if they notified that they will not be attending. Therefore, the wedding planner will assume that no one from these 50 invitations will attend. The wedding planner estimates that the each of the 25 guests planning to come solo has a 74% chance of attending alone, a 20% chance of not attending, and a 6% chance of bringing a companion. For each of the 60 RSVPs who plan to bring a companion, there is a 90% chance that they will…arrow_forward
- Prove that f: f →> R 16 One-to- one.arrow_forwardUse mathematical induction to prove the following statement: For all natural numbers n, 5 divides 6^n - 1 (show every step in detail)arrow_forwardUse mathematical induction to prove the following statement: For all natural numbers n, 5 divides 6^n - 1arrow_forward
- the set of all preimages of 2 isarrow_forwardWhich diagram(s) represent the following relationships An injective function from A to B? A surjective function from A to B? An injective function from B to A? A surjective function from B to A?arrow_forwardDetermine if each statement is true or false. If the statement is false, provide a brief explanation: a) There exists x = R such that √x2 = -x. b) Let A = {x = ZIx = 1 (mod 3)} and B = {x = ZIx is odd}. Then A and B are disjoint. c) Let A and B be subsets of a universal set U. If x = A and x/ € A - B,then x = An B.| E d) Let f : RR be defined by f (x) = 1 x + 2 1. Then f is surjective.arrow_forward
- Algebra & Trigonometry with Analytic GeometryAlgebraISBN:9781133382119Author:SwokowskiPublisher:Cengage
 Functions and Change: A Modeling Approach to Coll...AlgebraISBN:9781337111348Author:Bruce Crauder, Benny Evans, Alan NoellPublisher:Cengage Learning
Functions and Change: A Modeling Approach to Coll...AlgebraISBN:9781337111348Author:Bruce Crauder, Benny Evans, Alan NoellPublisher:Cengage Learning Linear Algebra: A Modern IntroductionAlgebraISBN:9781285463247Author:David PoolePublisher:Cengage Learning
Linear Algebra: A Modern IntroductionAlgebraISBN:9781285463247Author:David PoolePublisher:Cengage Learning


