
Connect for Chemistry
13th Edition
ISBN: 9781260161854
Author: Raymond Chang, Jason Overby
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.91QP
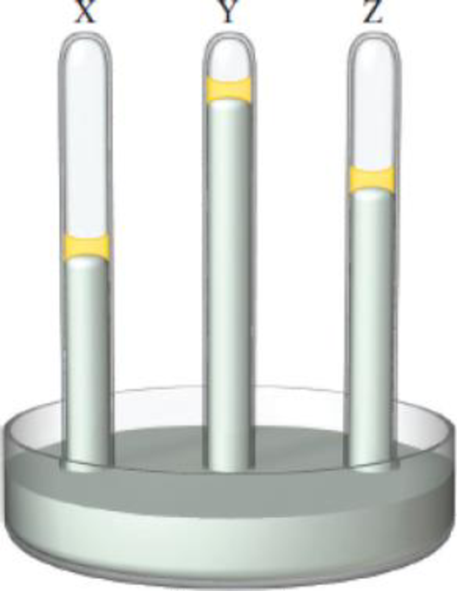
Consider the three mercury manometers shown. One of them has 1 mL of water on top of the mercury, another has 1 mL of a 1 m urea solution on top of the mercury, and the third one has 1 mL of a 1 m NaCl solution placed on top of the mercury. Which of these solutions is in the tube labeled X, which is in Y, and which is in Z?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than
one anomer, you can draw any of them.
Click and drag to start drawing a
structure.
X
Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the
mechanism of epoxide opening in aqueous acid.
2nd attempt
Be sure to show all four bonds at stereocenters using hash and wedge lines.
0
0
Draw curved arrows to show how the epoxide reacts with hydronium ion.
100 +1:
1st attempt
Feedback
Be sure to show all four bonds at stereocenters using hash and wedge lines.
See Periodic Table
See Hint
H
A
5
F
F
Hr
See Periodic Table See Hint
03 Question (1 point)
For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry.
>
1. CH₂CH₂MgBr
2. H₂O
3rd attempt
Draw all four bonds at chiral centers. Draw all stereoisomers formed.
Draw the structures here.
e
130
AN
H
See Periodic Table See Hint
P
C
Br
Chapter 12 Solutions
Connect for Chemistry
Ch. 12.2 - Prob. 1PECh. 12.2 - Prob. 1RCFCh. 12.2 - What is the strongest type of intermolecular force...Ch. 12.3 - Prob. 2PECh. 12.3 - Prob. 3PECh. 12.3 - Prob. 4PECh. 12.3 - Prob. 5PECh. 12.3 - A solution is prepared at 20C and its...Ch. 12.3 - Determine the percent composition by mass of LiCl...Ch. 12.3 - Prob. 3RCF
Ch. 12.4 - Using Figure 12.3, rank the potassium salts in...Ch. 12.5 - Calculate the molar concentration of oxygen in...Ch. 12.5 - Which of the following gases has the greatest...Ch. 12.5 - Prob. 2RCFCh. 12.6 - Calculate the vapor pressure of a solution made by...Ch. 12.6 - Prob. 8PECh. 12.6 - Prob. 9PECh. 12.6 - A solution of 0.85 g of an organic compound in...Ch. 12.6 - Prob. 11PECh. 12.6 - A solution contains equal molar amounts of liquids...Ch. 12.6 - What does it mean when we say that the osmotic...Ch. 12.6 - Calculate the boiling point and freezing point of...Ch. 12.7 - The freezing-point depression of a 0.100 m MgSO4...Ch. 12.7 - Indicate which compound in each of the following...Ch. 12.7 - Prob. 2RCFCh. 12 - Prob. 12.1QPCh. 12 - Prob. 12.2QPCh. 12 - Briefly describe the solution process at the...Ch. 12 - Prob. 12.4QPCh. 12 - Prob. 12.5QPCh. 12 - As you know, some solution processes are...Ch. 12 - Prob. 12.7QPCh. 12 - Describe the factors that affect the solubility of...Ch. 12 - Prob. 12.9QPCh. 12 - Prob. 12.10QPCh. 12 - Arrange the following compounds in order of...Ch. 12 - Explain the variations in solubility in water of...Ch. 12 - Prob. 12.13QPCh. 12 - Prob. 12.14QPCh. 12 - Calculate the percent by mass of the solute in...Ch. 12 - Calculate the amount of water (in grams) that must...Ch. 12 - Prob. 12.17QPCh. 12 - Prob. 12.18QPCh. 12 - Calculate the molalities of the following aqueous...Ch. 12 - For dilute aqueous solutions in which the density...Ch. 12 - Prob. 12.21QPCh. 12 - The concentrated sulfuric acid we use in the...Ch. 12 - Prob. 12.23QPCh. 12 - The density of an aqueous solution containing 10.0...Ch. 12 - Prob. 12.25QPCh. 12 - Describe the fractional crystallization process...Ch. 12 - A 3.20-g sample of a salt dissolves in 9.10 g of...Ch. 12 - The solubility of KNO3 is 155 g per 100 g of water...Ch. 12 - Prob. 12.29QPCh. 12 - Discuss the factors that influence the solubility...Ch. 12 - Prob. 12.31QPCh. 12 - Prob. 12.32QPCh. 12 - Prob. 12.33QPCh. 12 - A man bought a goldfish in a pet shop. Upon...Ch. 12 - A beaker of water is initially saturated with...Ch. 12 - A miner working 260 m below sea level opened a...Ch. 12 - The solubility of CO2 in water at 25C and 1 atm is...Ch. 12 - The solubility of N2 in blood at 37C and at a...Ch. 12 - Prob. 12.39QPCh. 12 - Write the equation representing Raoults law, and...Ch. 12 - Prob. 12.41QPCh. 12 - Prob. 12.42QPCh. 12 - Prob. 12.43QPCh. 12 - Prob. 12.44QPCh. 12 - Prob. 12.45QPCh. 12 - Prob. 12.46QPCh. 12 - Prob. 12.47QPCh. 12 - Describe how you would use freezing-point...Ch. 12 - Prob. 12.49QPCh. 12 - Prob. 12.50QPCh. 12 - The vapor pressure of benzene is 100.0 mmHg at...Ch. 12 - The vapor pressures of ethanol (C2H5OH) and...Ch. 12 - The vapor pressure of ethanol (C2H5OH) at 20C is...Ch. 12 - Prob. 12.54QPCh. 12 - What are the boiling point and freezing point of a...Ch. 12 - Prob. 12.56QPCh. 12 - Pheromones are compounds secreted by the females...Ch. 12 - The elemental analysis of an organic solid...Ch. 12 - How many liters of the antifreeze ethylene glycol...Ch. 12 - Prob. 12.60QPCh. 12 - Prob. 12.61QPCh. 12 - A solution of 2.50 g of a compound having the...Ch. 12 - A solution containing 0.8330 g of a polymer of...Ch. 12 - Prob. 12.65QPCh. 12 - A solution of 6.85 g of a carbohydrate in 100.0 g...Ch. 12 - Prob. 12.67QPCh. 12 - Prob. 12.69QPCh. 12 - Consider two aqueous solutions, one of sucrose...Ch. 12 - Arrange the following solutions in order of...Ch. 12 - Prob. 12.72QPCh. 12 - What are the normal freezing points and boiling...Ch. 12 - At 25C the vapor pressure of pure water is 23.76...Ch. 12 - Both NaCl and CaCl2 are used to melt ice on roads...Ch. 12 - A 0.86 percent by mass solution of NaCl is called...Ch. 12 - Prob. 12.77QPCh. 12 - Calculate the osmotic pressure of a 0.0500 M MgSO4...Ch. 12 - Prob. 12.79QPCh. 12 - Prob. 12.80QPCh. 12 - Prob. 12.81QPCh. 12 - Water and methanol are miscible with each other...Ch. 12 - Lysozyme is an enzyme that cleaves bacterial cell...Ch. 12 - Prob. 12.84QPCh. 12 - Prob. 12.85QPCh. 12 - Two liquids A and B have vapor pressures of 76...Ch. 12 - Prob. 12.87QPCh. 12 - Prob. 12.88QPCh. 12 - Prob. 12.89QPCh. 12 - Calculate the mass of naphthalene (C10H8) that...Ch. 12 - Consider the three mercury manometers shown. One...Ch. 12 - Prob. 12.92QPCh. 12 - Prob. 12.93QPCh. 12 - A solution of 1.00 g of anhydrous aluminum...Ch. 12 - Desalination is a process of removing dissolved...Ch. 12 - Prob. 12.96QPCh. 12 - A protein has been isolated as a salt with the...Ch. 12 - Prob. 12.98QPCh. 12 - Hydrogen peroxide with a concentration of 3.0...Ch. 12 - State which of the alcohols listed in Problem...Ch. 12 - Prob. 12.101QPCh. 12 - Iodine (I2) is only sparingly soluble in water...Ch. 12 - Prob. 12.103QPCh. 12 - In the apparatus shown, what will happen if the...Ch. 12 - Prob. 12.105QPCh. 12 - Concentrated hydrochloric acid is usually...Ch. 12 - Explain each of the following statements: (a) The...Ch. 12 - Prob. 12.108QPCh. 12 - A 0.050 M hydrofluoric acid (HF) solution is 11...Ch. 12 - Shown here is a plot of vapor pressures of two...Ch. 12 - Prob. 12.111QPCh. 12 - Prob. 12.112QPCh. 12 - Prob. 12.113QPCh. 12 - Prob. 12.114QPCh. 12 - Prob. 12.115QPCh. 12 - A mixture of ethanol and 1-propanol behaves...Ch. 12 - Prob. 12.117QPCh. 12 - Prob. 12.118QPCh. 12 - Prob. 12.119QPCh. 12 - Acetic acid is a weak acid that ionizes in...Ch. 12 - Making mayonnaise involves beating oil into small...Ch. 12 - Acetic acid is a polar molecule and can form...Ch. 12 - A 2.6-L sample of water contains 192 g of lead....Ch. 12 - Certain fishes in the Antarctic Ocean swim in...Ch. 12 - Prob. 12.125QPCh. 12 - Prob. 12.126QPCh. 12 - Prob. 12.127QPCh. 12 - At 27C, the vapor pressure of pure water is 23.76...Ch. 12 - Prob. 12.129QPCh. 12 - Liquids A (molar mass 100 g/mol) and B (molar mass...Ch. 12 - A very long pipe is capped at one end with a...Ch. 12 - Prob. 12.132QPCh. 12 - A mixture of liquids A and B exhibits ideal...Ch. 12 - Prob. 12.134QPCh. 12 - (a) Derive the equation relating the molality (m)...Ch. 12 - Prob. 12.136QPCh. 12 - A student carried out the following procedure to...Ch. 12 - Valinomycin is an antibiotic. It functions by...Ch. 12 - Prob. 12.139QPCh. 12 - Here is an after-dinner trick. With guests still...Ch. 12 - The molecule drawn here has shown promise as an...Ch. 12 - The Henrys law constant of oxygen in water at 25C...Ch. 12 - The diagram shows the vapor pressure curves for...Ch. 12 - Prob. 12.144QPCh. 12 - Prob. 12.146QP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forward
- Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forward
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY