
(a)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkene used to synthesize the given compound is
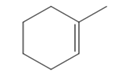
The necessary reagents and special reaction conditions for the synthesis are
Explanation of Solution
The structure of the given compound is
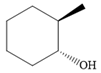
Alcohol with a specific stereochemistry is to be synthesized from an alkene. A reaction involving a carbocation needs to be avoided to prevent unwanted carbocation rearrangements. Also, the OH group must be added to a less substituted carbon, i.e., an anti-Markovnikov addition is needed. Therefore, the reaction needs to be carried out using hydroboration-oxidation.
The appropriate alkene for the synthesis of the given compound would be
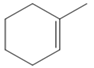
The given compound is synthesized by using the above alkene via hydroboration-oxidation reaction. So the necessary reagents for the reaction are
In the first step, an electrophilic addition of borane across the double bond of the alkene takes place either from above or below the plane of the alkene. So a mixture of enantiomers is obtained after oxidation of the adduct by basic
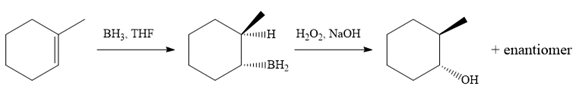
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
(b)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of alkenes and alkynes is carried out by the hydroboration-oxidation reaction. Alkenes are oxidized to alcohol while alkynes are oxidized to the corresponding carbonyl compounds. Terminal alkynes are oxidized to the corresponding aldehyde, and internal alkynes are oxidized to the corresponding ketone by a sterically hindered borane like disiamylborane
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkyne used to synthesize the given compound is
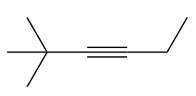
The necessary reagents and special reaction conditions to synthesize the given compound are
Explanation of Solution
The given compound is
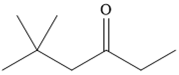
It is a ketone, so the starting compound must be an alkyne. A hydroboration-oxidation reaction can convert an alkyne into a ketone. Since only one molecule of borane is to be added, a bulky reagent like disiamylborane is more appropriate than borane. Also, the dialkylborane part must add to the less hindered carbon of the triple bond. Therefore, the triple bond must be between the carbon bonded to oxygen and the carbon close to the bulky tertiary carbon. Therefore, the alkyne that can be used is

A sterically hindered dialkylborane, like disiamylborane
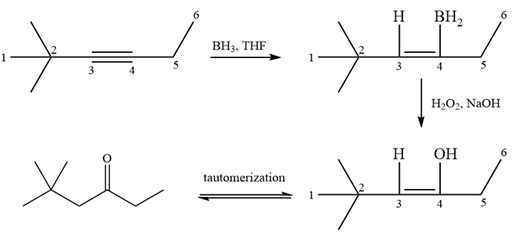
Thus the specific reagents and reaction conditions are
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
(c)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of alkenes and alkynes is carried out by the hydroboration-oxidation reaction. Alkenes are oxidized to alcohol while alkynes are oxidized to the corresponding carbonyl compounds. Terminal alkynes are oxidized to the corresponding aldehyde, and internal alkynes are oxidized to the corresponding ketone by a sterically hindered borane like disiamylborane
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkyne used to synthesize the given compound is
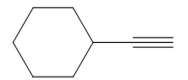
The necessary reagents and special reaction conditions to synthesize the given compound are
Explanation of Solution
The given compound is
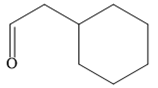
It is an aldehyde, so it can be prepared from a terminal alkyne by hydroboration-oxidation. In the hydroboration reaction, boron is added to the terminal carbon. So the appropriate alkyne for the synthesis of the given compound is
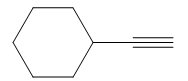
The alkyne is treated with the bulky disiamylborane to prevent the addition of a second molecule and formation of a mixture of products. Subsequent treatment of the adduct by
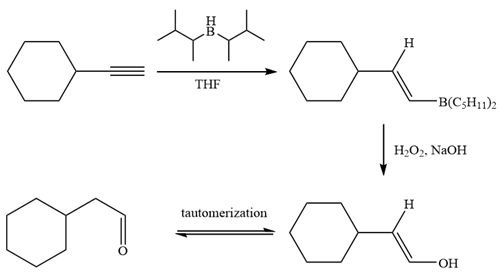
Thus, the necessary reagents and special reaction conditions for the synthesis are
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
(d)
Interpretation:
The appropriate alkene or alkyne from which the given compound can be produced is to be determined along with the necessary reagents and special reaction conditions.
Concept introduction:
Oxidation of alkenes and alkynes is carried out by the hydroboration-oxidation reaction. Alkenes are oxidized to alcohol while alkynes are oxidized to the corresponding carbonyl compounds. Terminal alkynes are oxidized to the corresponding aldehyde, and internal alkynes are oxidized to the corresponding ketone by a sterically hindered borane like disiamylborane
The initial addition of borane is a syn, anti-Markovnikov addition via a four-membered cyclic transition state. Boron adds to the less substituted (less hindered) carbon because it is less electronegative than hydrogen. This results in an anti-Markovnikov addition of a water molecule to the double bond.
Answer to Problem 12.53P
The appropriate alkene used to synthesize the given compound is
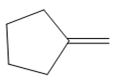
The necessary reagents and special reaction conditions for the synthesis are
Explanation of Solution
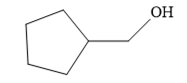
Since the product is an alcohol, an alkene with a methylene substituent on a cyclopentane ring would be appropriate as the starting compound.

Treating this alkene with borane in THF will add
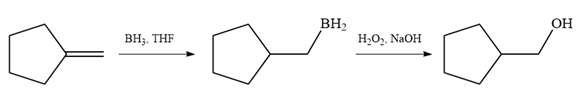
Thus, the necessary reagents for the reaction are
The alkene necessary for the synthesis of the given compound and the reagents and special conditions for the reaction are determined on the basis of the structure of the given compound.
Want to see more full solutions like this?
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





