
(a)
Interpretation:
The detailed mechanism with the major product for the given reaction is to be drawn.
Concept introduction:
The acid-catalyzed hydration of an
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
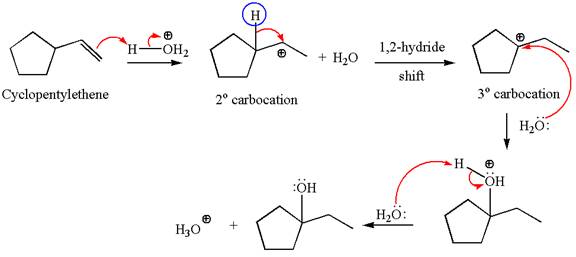
Explanation of Solution
The given reaction is
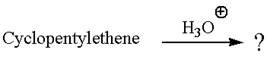
Cyclopentylethene, a terminal alkene, in presence of
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
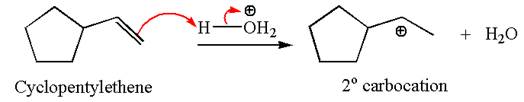
The secondary carbocation can be rearranged to a more stable tertiary carbocation by
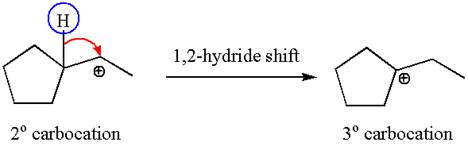
In the second step, the water molecule acts as a nucleophile and attacks the tertiary carbocation, forming a tertiary alcohol product, followed by deprotonation of the positively charged oxygen.
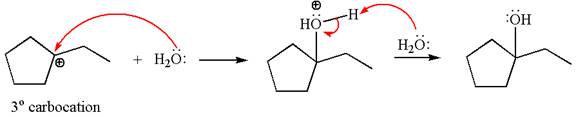
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurs through carbocation rearrangement.
(b)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The oxymercuration-reduction is also the reaction of addition of water across the
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
Explanation of Solution
The given reaction is
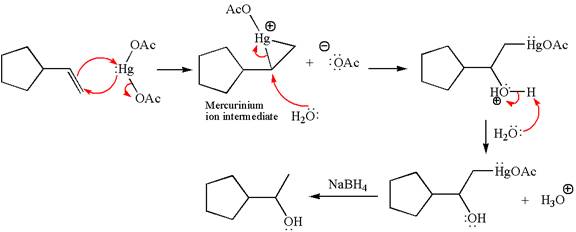
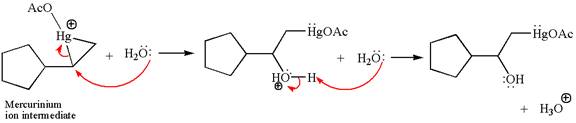
Cyclopentylethene, a terminal alkene, on reaction with mercury
In the first step, the electron rich
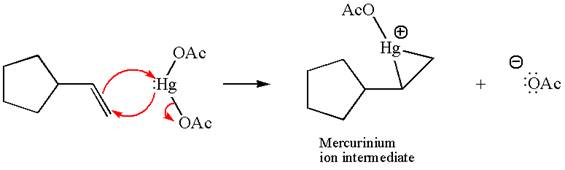
In the second step, the water molecule acts as a nucleophile and, according to Markovnikov rule, attacks the most substituted side to open the three-membered ring, followed by deprotonation of the positively charged oxygen atom.
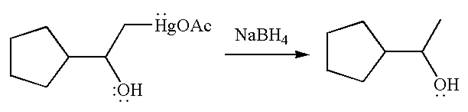
The product formed in the previous step is then subjected to reduction with sodium borohydride,
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through formation of mercurinium ion intermediate without rearrangement.
(c)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The acid-catalyzed hydration of an alkene is the electrophilic addition of water across the
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
Explanation of Solution
The given reaction is
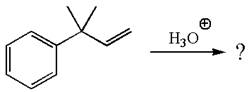
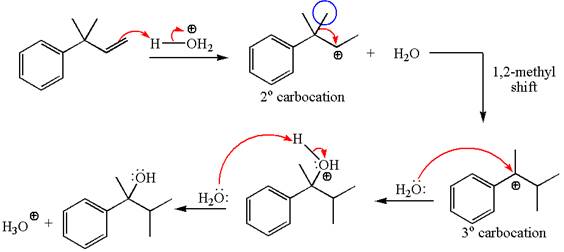
The given substrate, a terminal alkene, in the presence of
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
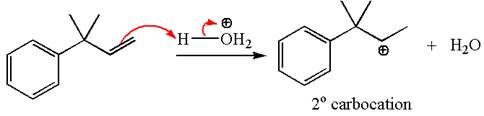
The secondary carbocation can be rearranged to a more stable tertiary as well as resonance stabilized carbocation by
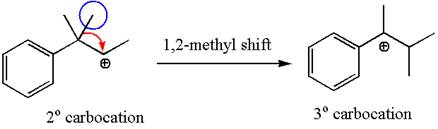
In the second step, the water molecule acts as a nucleophile and attacks the tertiary carbocation, forming a tertiary alcohol product, followed by deprotonation of the positively charged oxygen.
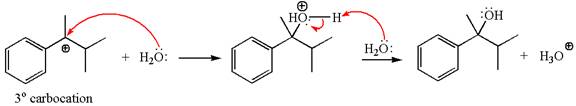
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through carbocation rearrangement.
(d)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The oxymercuration-reduction is also the reaction of addition of water across the
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
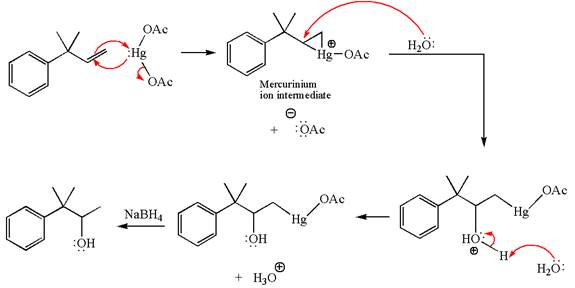
Explanation of Solution
The given reaction is
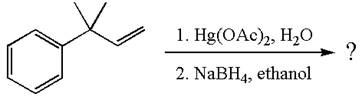
The given substrate, a terminal alkene, on reaction with mercury
In the first step, the electron rich
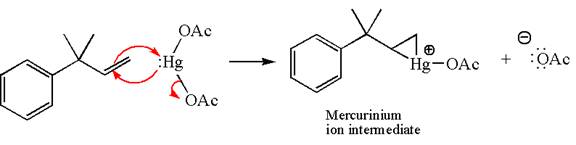
In the second step, the water molecule acts as a nucleophile and, according to Markovnikov rule, attacks the most substituted side to open the three-membered ring, followed by deprotonation of the positively charged oxygen atom.
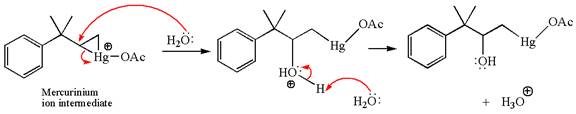
The product formed in the previous step is then subjected to reduction with sodium borohydride,
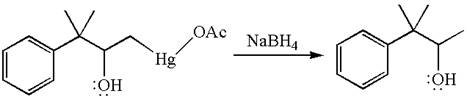
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through formation of mercurinium ion intermediate without rearrangement.
Want to see more full solutions like this?
Chapter 12 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- What are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forwardPotential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forward
- Can u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
