
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
9th Edition
ISBN: 9780135213728
Author: Leroy Wade, Jan Simek
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.24SP
covered a synthesis of
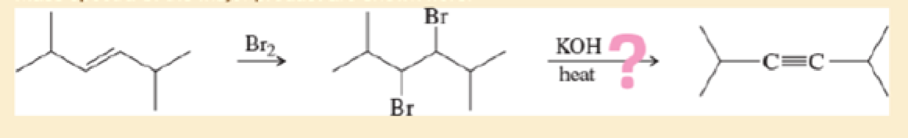
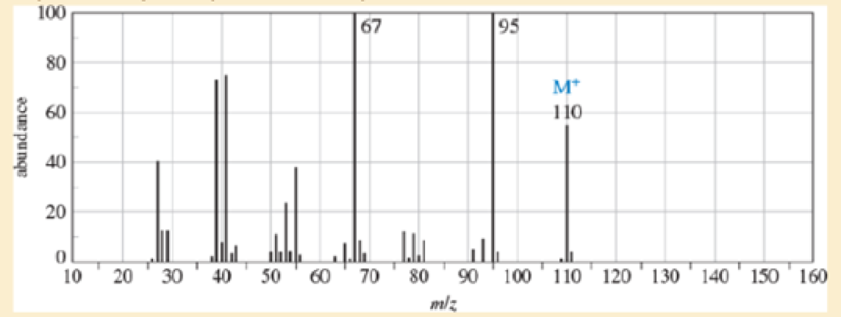
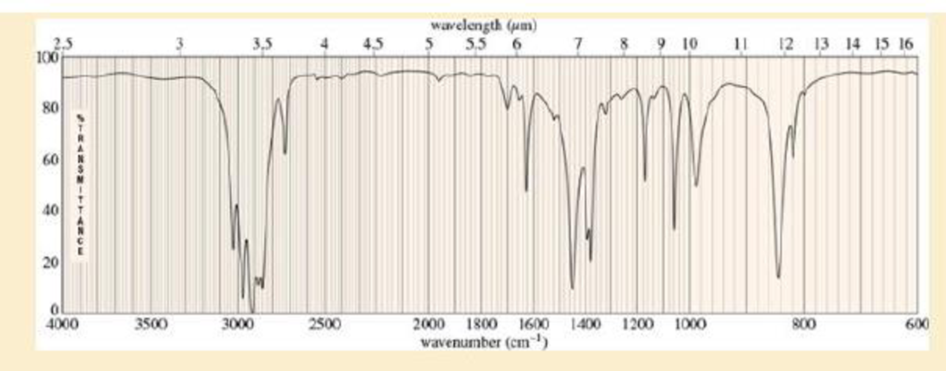
- a. Do the spectra confirm the right product? If not, what is it?
- b. Explain the important peaks in the IR spectrum.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
b)
8.
Indicate whether the following carbocation rearrangements are likely to occur
Please explain your rational using 10 words or less
not likely to occur
• The double bond is still in the
Same position
+
Likely
to oc
occur
WHY?
-3
H3C
Brave
Chair Conformers. Draw the chair conformer of the following substituted
cyclohexane. Peform a RING FLIP and indicate the most stable
conformation and briefly explain why using 20 words or less.
CI
2
-cobs ??
MUST INDICATE H -2
-2
Br
EQ
Cl
OR
AT
Br
H&
most stable
WHY?
- 4
CH
12
Conformational Analysis. Draw all 6 conformers (one above each letter) of the
compound below looking down the indicated bond. Write the letter of the
conformer with the HIGHEST and LOWEST in energies on the lines provided.
NOTE: Conformer A MUST be the specific conformer of the structure as drawn below
-4 NOT
HOH
OH
3
Conformer A:
Br
OH
A
Samo
Br H
04
Br
H
H3
CH₂
H
anti
stagere
Br CH
clipsed
H
Brott
H
IV
H
MISSING 2
-2
B
C
D
E
F
X
6
Conformer with HIGHEST ENERGY:
13. (1
structure
LOWEST ENERGY:
Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the
corresponding to this name. HINT: Do not forget to indicate stereochemistry
when applicable.
a)
८८
2
"Br
{t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa
Parent name (noname)
4 Bromo
Sub = 2-methylethyl-4 Bromo nonane
b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane
# -2
-2
in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!
Chapter 12 Solutions
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
Give the IUPAC name for each compound.
Organic Chemistry
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forwardIndicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forward
- The molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forwardWhat characteristics should an interface that forms an electrode have?arrow_forward
- For a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forwardDescribe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License