
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.23SP
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.
- a. Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?
- b. Use the IR spectrum to determine the
functional group (s), if any. - c. Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew that hydrogenation of the compound gives n-octane, would the structure still be uncertain?
- d. Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum.
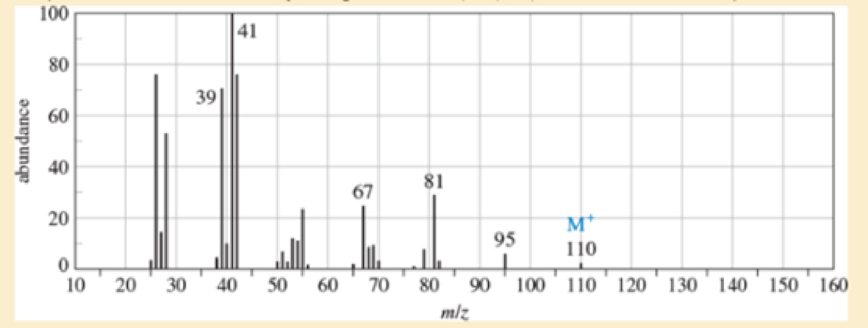
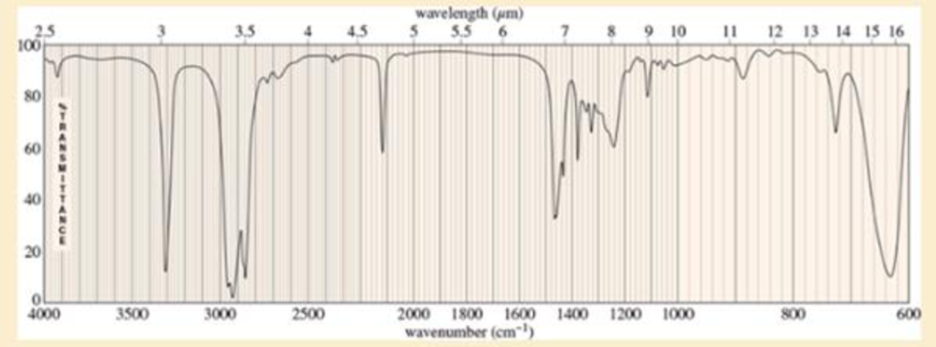
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
QUESTION: Fill in the answers in the empty green boxes
1. Step 2
2. Step 3
3. Step 4 (SUM)
4. Step 5 (df) (GIVEN)
5. Determine S y/x value
*The data values have been provided in the worksheet attached in the first image*
If the symbol A is placed in a reaction, at what temperature does it take place?
By malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).
Chapter 12 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY