
Concept explainers
(a)
Interpretation:
Equation for hydration of given

Concept Introduction:
Addition of water molecule to an unsaturated bond present in hydrocarbon is known as hydration. During hydration, the unsaturated bond present is broken and a carbon‑hydrogen, carbon‑hydroxyl bond is formed.
General structure of alkene can be given as,

Alkene on hydration gives alcohol. As there is only one unsaturated bond present in alkene, only one molecule of water is consumed. The general equation for the hydration of alkene can be given as,
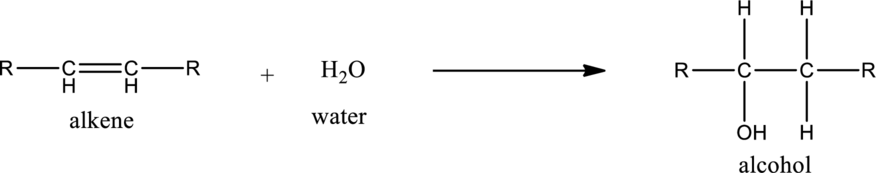
If the alkene is symmetrical only one product is formed. If the alkene is unsymmetrical, then the product is formed according the Markovnikov’s rule. According to this rule, in hydration reaction, the hydrogen atom gets attached to the carbon atom in the unsaturated bond which has more number of hydrogen atoms or less number of substituents.
(b)
Interpretation:
Equation for hydration of given alkene has to be written and the major product has to be predicted.
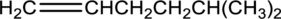
Concept Introduction:
Refer part (a).
(c)
Interpretation:
Equation for hydration of given alkene has to be written and the major product has to be predicted.
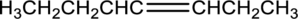
Concept Introduction:
Refer part (a).
(d)
Interpretation:
Equation for hydration of given alkene has to be written and the major product has to be predicted.

Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Connect 2-Year Online Access for General, Organic, and Biochemistry
- Consider the redox reaction: 2 P4 + 8 OH− + 4 H2O → 4 PH3 + 4 HPO32− The element oxidized is ["", "", ""] , the element reduced is ["", "", ""] , one of the oxidizing agents is ["", "", ""] , and the reducing agent is ["", "", ""] .arrow_forwardWhat is the missing reactant in this organic reaction? OH H + R Δ CH3-CH2-CH-CH3 O CH3 CH3-CH2-C-O-CH-CH2-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answe box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C O2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cerarrow_forwardPredict the product of this organic reaction: CH3 NH2 Δ CH3-CH-CH3 + HO-C-CH2-N-CH3 P+H₂O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. Xarrow_forward
- In the scope of the SCH4U course, please thoroughly go through the second questionarrow_forwardPlease help me solve these two problems. Thank you in advance.arrow_forwardNaming and drawing unsubstituted esters Write the systematic name of each organic molecule: Explanation structure Check name Х 2/5arrow_forward
- Predict the product of this organic reaction: =0 CH3-O-CH2-C-OH + CH3-OH H P+H₂O A Specifically, in the drawing area below draw the condensed structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. ☐arrow_forwardNaming and drawing USUsted ester Draw the condensed structure of ethyl hexanoate. Click anywhere to draw the first atom of your structure. × A : ☐arrow_forwardExtra for Experts: Your Future in Chemistry. As you now know, there are countless jobs that involve chemistry! Research a chemistry profession that interests you. In your answer, discuss which aspects of the job most appeal to you.arrow_forward
- MISSED THIS? Read Section 19.9 (Pages 878-881); Watch IWE 19.10 Consider the following reaction: CH3OH(g) CO(g) + 2H2(g) (Note that AG,CH3OH(g) = -162.3 kJ/mol and AG,co(g)=-137.2 kJ/mol.) Part A Calculate AG for this reaction at 25 °C under the following conditions: PCH₂OH Pco PH2 0.815 atm = 0.140 atm 0.170 atm Express your answer in kilojoules to three significant figures. Ο ΑΣΦ AG = -150 Submit Previous Answers Request Answer □? kJ × Incorrect; Try Again; 2 attempts remaining Calculate the free energy change under nonstandard conditions (AGrxn) by using the following relationship: AGrxn = AGrxn + RTInQ, AGxn+RTInQ, where AGxn is the standard free energy change, R is the ideal gas constant, T is the temperature in kelvins, a is the reaction quotient. Provide Feedback Next >arrow_forwardIdentify and provide a brief explanation of Gas Chromatography (GC) within the context of chemical analysis of food. Incorporate the specific application name, provide a concise overview of sample preparation methods, outline instrumental parameters and conditions ultilized, and summarise the outcomes and findings achieved through this analytical approach.arrow_forwardIdentify and provide a concise explanation of the concept of signal-to-noise ratio (SNR) in the context of chemical analysis. Provide specific examples.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





