
CHEMISITRY W/OWL PKG LOOSELEAF
9th Edition
ISBN: 9781285903859
Author: ZUMDAHL
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 91AE
The solubility of benzoic acid (HC7H5O2),
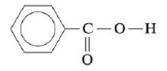
is 0.34 g/100 mL in water at 25°C and is 10.0 g/100 mL in benzene (C6H6) at 25°C. Rationalize this solubility behavior. (Hint: Benzoic acid forms a dimer in benzene.) Would benzoic acid be more or less soluble in a 0.1-M NaOH solution than it is in water? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area.
HO-
HO-
-0
OH
OH
HO
NG
HO-
HO-
OH
OH
OH
OH
NG
OH
€
+
Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn
it into the product of the reaction.
Also, write the name of the product molecule under the drawing area.
Name: ☐
H
C=0
X
H-
OH
HO-
H
HO-
-H
CH₂OH
×
Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than
one anomer, you can draw any of them.
Click and drag to start drawing a
structure.
X
Chapter 11 Solutions
CHEMISITRY W/OWL PKG LOOSELEAF
Ch. 11 - Prob. 1RQCh. 11 - Using KF as an example, write equations that refer...Ch. 11 - Prob. 3RQCh. 11 - Prob. 4RQCh. 11 - Define the terms in Raoults law. Figure 10-9...Ch. 11 - In terms of Raoults law, distinguish between an...Ch. 11 - Vapor-pressure lowering is a colligative property,...Ch. 11 - What is osmotic pressure? How is osmotic pressure...Ch. 11 - Distinguish between a strong electrolyte, a weak...Ch. 11 - Prob. 10RQ
Ch. 11 - Prob. 1ALQCh. 11 - Once again, consider Fig. 10-9. Suppose instead of...Ch. 11 - Prob. 3ALQCh. 11 - Prob. 4ALQCh. 11 - You have read that adding a solute to a solvent...Ch. 11 - You drop an ice cube (made from pure water) into a...Ch. 11 - Using the phase diagram for water and Raoults law,...Ch. 11 - You and your friend are each drinking cola from...Ch. 11 - Prob. 9ALQCh. 11 - Prob. 10ALQCh. 11 - Rubbing alcohol contains 585 g isopropanol...Ch. 11 - Prob. 12SRCh. 11 - Prob. 13SRCh. 11 - Prob. 14SRCh. 11 - Calculate the sodium ion concentration when 70.0...Ch. 11 - Write equations showing the ions present after the...Ch. 11 - Rationalize the temperature dependence of the...Ch. 11 - The weak electrolyte NH3(g) does not obey Henrys...Ch. 11 - The two beakers in the sealed container...Ch. 11 - The following plot shows the vapor pressure of...Ch. 11 - When pure methanol is mixed with water, the...Ch. 11 - Prob. 22QCh. 11 - For an acid or a base, when is the normality of a...Ch. 11 - Prob. 24QCh. 11 - Prob. 25QCh. 11 - Prob. 26QCh. 11 - Explain the terms isotonic solution, crenation,...Ch. 11 - Prob. 28QCh. 11 - Prob. 29ECh. 11 - Prob. 30ECh. 11 - Common commercial acids and bases are aqueous...Ch. 11 - In lab you need to prepare at least 100 mL of each...Ch. 11 - Prob. 33ECh. 11 - Prob. 34ECh. 11 - Prob. 35ECh. 11 - Calculate the molarity and mole fraction of...Ch. 11 - Prob. 37ECh. 11 - Prob. 38ECh. 11 - Prob. 39ECh. 11 - a. Use the following data to calculate the...Ch. 11 - Although Al(OH)3 is insoluble in water, NaOH is...Ch. 11 - Prob. 42ECh. 11 - Prob. 43ECh. 11 - Prob. 44ECh. 11 - For each of the following pairs, predict which...Ch. 11 - Which ion in each of the following pairs would you...Ch. 11 - Rationalize the trend in water solubility for the...Ch. 11 - In flushing and cleaning columns used in liquid...Ch. 11 - The solubility of nitrogen in water is 8.21 104...Ch. 11 - Calculate the solubility of O2 in water at a...Ch. 11 - Glycerin, C3H8O3, is a nonvolatile liquid. What is...Ch. 11 - The vapor pressure of a solution containing 53.6 g...Ch. 11 - The normal boiling point of diethyl ether is...Ch. 11 - At a certain temperature, the vapor pressure of...Ch. 11 - A solution is made by dissolving 25.8 g urea...Ch. 11 - A solution of sodium chloride in water has a vapor...Ch. 11 - Prob. 57ECh. 11 - A solution is prepared by mixing 0.0300 mole of...Ch. 11 - What is the composition of a methanol...Ch. 11 - Benzene and toluene form an ideal solution....Ch. 11 - Which of the following will have the lowest total...Ch. 11 - Prob. 62ECh. 11 - Match the vapor pressure diagrams with the...Ch. 11 - The vapor pressures of several solutions of...Ch. 11 - A solution is prepared by dissolving 27.0 g urea,...Ch. 11 - A 2.00-g sample of a large biomolecule was...Ch. 11 - What mass of glycerin (C3H8O3), a nonelectrolyte,...Ch. 11 - The freezing point of 1-butanol is 25.50C and Kf...Ch. 11 - Prob. 69ECh. 11 - What volume of ethylene glycol (C2H6O2), a...Ch. 11 - Reserpine is a natural product isolated from the...Ch. 11 - A solution contains 3.75 g of a nonvolatile pure...Ch. 11 - a. Calculate the freezing-point depression and...Ch. 11 - Erythrocytes are red blood cells containing...Ch. 11 - Prob. 75ECh. 11 - Prob. 76ECh. 11 - Prob. 77ECh. 11 - Prob. 78ECh. 11 - Consider the following solutions: 0.010 m Na3PO4...Ch. 11 - From the following: pure water solution of...Ch. 11 - Prob. 81ECh. 11 - Prob. 82ECh. 11 - Prob. 83ECh. 11 - Consider the following representations of an ionic...Ch. 11 - Prob. 85ECh. 11 - Prob. 86ECh. 11 - Use the following data for three aqueous solutions...Ch. 11 - The freezing-point depression of a 0.091-m...Ch. 11 - Prob. 89ECh. 11 - A 0.500-g sample of a compound is dissolved in...Ch. 11 - The solubility of benzoic acid (HC7H5O2), is 0.34...Ch. 11 - Prob. 92AECh. 11 - In Exercise 96 in Chapter 8, the pressure of CO2...Ch. 11 - Explain the following on the basis of the behavior...Ch. 11 - The term proof is defined as twice the percent by...Ch. 11 - Prob. 97AECh. 11 - Prob. 98AECh. 11 - A solution is made by mixing 50.0 g acetone...Ch. 11 - Prob. 100AECh. 11 - Thyroxine, an important hormone that controls the...Ch. 11 - Prob. 102AECh. 11 - An unknown compound contains only carbon,...Ch. 11 - Prob. 104AECh. 11 - Prob. 105AECh. 11 - Prob. 106AECh. 11 - Prob. 107AECh. 11 - Prob. 108AECh. 11 - Patients undergoing an upper gastrointestinal...Ch. 11 - Prob. 110CWPCh. 11 - The lattice energy of NaCl is 786 kJ/mol, and the...Ch. 11 - For each of the following pairs, predict which...Ch. 11 - The normal boiling point of methanol is 64.7C. A...Ch. 11 - A solution is prepared by mixing 1.000 mole of...Ch. 11 - Prob. 115CWPCh. 11 - A 4.7 102 mg sample of a protein is dissolved in...Ch. 11 - A solid consists of a mixture of NaNO3 and...Ch. 11 - The vapor pressure of pure benzene is 750.0 torr...Ch. 11 - Prob. 119CPCh. 11 - Plants that thrive in salt water must have...Ch. 11 - You make 20.0 g of a sucrose (C12H22O11) and NaCl...Ch. 11 - Prob. 122CPCh. 11 - The vapor in equilibrium with a pentane-hexane...Ch. 11 - A forensic chemist is given a white solid that is...Ch. 11 - A 1.60-g sample of a mixture of naphthalene...Ch. 11 - Prob. 126CPCh. 11 - Prob. 127CPCh. 11 - You have a solution of two volatile liquids, A and...Ch. 11 - In some regions of the southwest United States,...Ch. 11 - Creatinine, C4H7N3O, is a by-product of muscle...Ch. 11 - An aqueous solution containing 0.250 mole of Q, a...Ch. 11 - Anthraquinone contains only carbon, hydrogen, and...
Additional Science Textbook Solutions
Find more solutions based on key concepts
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward
- 1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forward
- Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forwardSelect the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward
- 5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forwardplease help fill in the tablearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY