
Organic Chemistry, Third Edition Binder Ready Version
3rd Edition
ISBN: 9781119110453
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 38CP
Interpretation Introduction
Interpretation:
A multi-step synthesis of E-2-hexenal from 1,1-dibromopentane has to be given.
Concept Introduction:
Hydroboration-oxidation reaction: Addition of
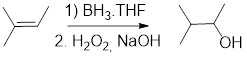
Ozonolysis: It involves breaking of
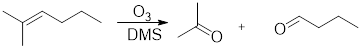
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name the following molecules using IUPAC.
Identify each alcohol in the molecule as either primary, secondary, or tertiary.
Draw the mechanism for the grignard reagent reactant.
Chapter 11 Solutions
Organic Chemistry, Third Edition Binder Ready Version
Ch. 11.1 - Prob. 1CCCh. 11.1 - Prob. 2CCCh. 11.2 - Prob. 1LTSCh. 11.2 - Prob. 3PTSCh. 11.2 - Prob. 4ATSCh. 11.3 - Prob. 2LTSCh. 11.3 - Prob. 5PTSCh. 11.3 - As we will see in Chapter 26, steroids are a class...Ch. 11.4 - Prob. 3LTSCh. 11.4 - Prob. 7PTS
Ch. 11.4 - Prob. 8ATSCh. 11.5 - Prob. 4LTSCh. 11.5 - Prob. 9PTSCh. 11.5 - Prob. 10ATSCh. 11 - Prob. 11PPCh. 11 - Prob. 12PPCh. 11 - Prob. 13PPCh. 11 - Prob. 14PPCh. 11 - Prob. 15PPCh. 11 - Prob. 16PPCh. 11 - Prob. 17PPCh. 11 - Prob. 18PPCh. 11 - Prob. 19PPCh. 11 - Prob. 20PPCh. 11 - Prob. 21PPCh. 11 - Prob. 22PPCh. 11 - Prob. 23PPCh. 11 - Prob. 24PPCh. 11 - Prob. 25PPCh. 11 - Prob. 26PPCh. 11 - Prob. 27IPCh. 11 - Prob. 28IPCh. 11 - Prob. 29IPCh. 11 - Prob. 30IPCh. 11 - Prob. 31IPCh. 11 - In a study of volatile compounds extracted from...Ch. 11 - Kakkon is the root of an Asian plant that has...Ch. 11 - Prob. 34IPCh. 11 - Prob. 35IPCh. 11 - Prob. 37IPCh. 11 - The aroma, taste, and general quality of wine are...Ch. 11 - Prob. 39CPCh. 11 - Prob. 40CPCh. 11 - Prob. 42CPCh. 11 - Prob. 43CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me figure out the mechanism with arrows of the following reactionarrow_forwardOrganic Functional Groups Predicting the reactants or products of acetal hydrolysis termine the structures of the missing organic molecules in the following reaction: H* H* + H₂O Y ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure Explanation Check @2 W Click and drag to start drawing a structure. #4 # 3 LU E % 67 olo 5 66 R T Y & 7 AcGraw Hill LLC. All Rights R Xarrow_forward8. (16 pts) Provide the stepwise mechanism for the synthesis of the following compound via an enaminearrow_forward
- Draw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY