
Hydrogen bromide is a highly reactive and corrosive gas used mainly as a catalyst for organic reactions. It is produced by reacting hydrogen and bromine gases together.
Â
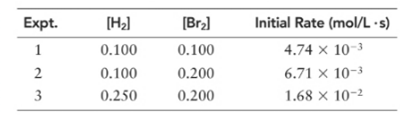
(a) What is the order of the reaction with respect to hydrogen, bromine, and overall?
(b) Write the rate expression of the reaction.
(c) Calculate k for the reaction. What are the units for k?
(d) When
(a)
Interpretation:
To determine the order of reaction with respect to BF3, NH3 and overall for the following reaction:
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Order of given reaction:
With respect to H2 =1
With respect to Br2 =
Overall =
Explanation of Solution
Given information:
Here the chemical reaction is:

Let’s assume the reaction to be ‘t’ order with respect to H2 and ‘y’ order with respect to Br2.
Then, rate law for experiment 1 in above reaction will be;
And, rate law for experiment 3 in above reaction will be;
Divide (1) by (2) to get value of ‘t’.
Thus, order with respect to Br2 is
Now, rate law for experiment 4 in above reaction will be;
Divide (2) by (3) to get value of ‘y’.
Thus, order with respect to H2 is 1.
And the order of reaction will be:
Thus, overall order of reaction is 2.
(b)
Interpretation:
To write the rate expression for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Rate law expression for above reaction will be;
Explanation of Solution
Here the chemical reaction is:
Order of reaction with respect to H2 = 2
Order of reaction with respect to Br2 =
Let the rate constant be ‘k’.
Then, rate law expression for above reaction will be;
(c)
Interpretation:
To determine the rate constant and its unit for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Rate constant is
Unit of rate constant is
Explanation of Solution
Here the chemical reaction is:
Writing rate law for experiment 1 in above reaction will be;
Hence, the rate constant is
And unit of rate constant is
(d)
Interpretation:
To determine the rate of reaction at given concentration of reactants.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Rate of reaction for given reaction at given conditions is
Explanation of Solution
Here the chemical reaction is:
Rate law expression for above reaction:
Here we have:
[H2 ]=0.421 M
[Br2 ] = 0.215 M
Rate constant =
Plugging values in rate law as:
Hence, the rate of reaction is
Want to see more full solutions like this?
Chapter 11 Solutions
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
- What I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forwardConsider the solubility products (Ksp values) for the following compounds:SrSO4 (Ksp = 7.6 x 10−7), BaSO4 (Ksp = 1.5 x 10−9), SrCO3 (Ksp = 7.0 x 10−10), BaCO3 (Ksp = 1.6 x 10−9)Which anion is the harder base, CO32− or SO42−? Justify your answer.arrow_forwardQ1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward
- (4 pts - 2 pts each part) A route that can be taken to prepare a hydrophobic (water-repellent) aerogel is to start with trichloromethylsilane, CH3SiCl3 as the silicon source. a. What is the chemical reaction that this undergoes to form a product with Si-OH groups? Write as complete of a chemical equation as you can. CI CI-SI-CH3 CI b. The formation of a byproduct is what drives this reaction - what is the byproduct (if you didn't already answer it in part (a)) and how/why does it form?arrow_forwardb) Circle the substrate that would not efficiently generate a Grignard reagent upon reaction with Mg in ether. CI Br ד c) Circle the Grignard reagents that contain incompatible functional groups. MgBr HO MgBr MgBr MgBr MgBr HO MgBrarrow_forwardQ2: Predict all organic product(s), including stereoisomers when applicable. PCC OH a) CH2Cl2 Page 2 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) .OH Na2Cr2O7, H+ OH PCC CH2Cl2 c) OHarrow_forward
- d) Circle the substrates that will give an achiral product after a Grignard reaction with CH3MgBr. Harrow_forwardQ4: Predict the organic products for the following reactions. Then draw curved arrow electron- pushing mechanism for the reactions. a) NaBH4 EtOH Page 4 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) 요 1. Et₂O H MgBr 2. H+, H₂Oarrow_forward5Helparrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





