
Concept explainers
Interpretation:
The density of a hypothetical ionic compound for given radii and masses of cations and anions, is to be determined.
Concept introduction:
Ionic compounds contain cations and anions that are arranged in a crystal lattice.
A crystal lattice is made of small repeating unit cells.
A unit cell is of primitive or centered type.
Each atom or ion in a unit cell is shared by the adjacent cells. An atom or ions at corners is shared by eight unit cells.
The edge length of a body-centered cubic cell is given by the relation as follows:
Here,
is the edge length,
is the radius of cation, and
The volume of the unit cell
The density
Here,
is the mass.
Answer to Problem 146AP
Solution:
Explanation of Solution
Given information:
Radii of the anion
The figure is as shown below:
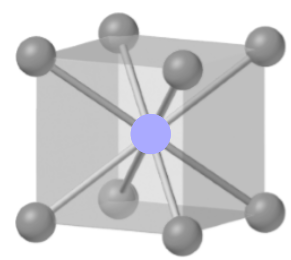
The given unit cell is body-centered, in which there is one cation at the center, and eight anions at the corners, shared by eight unit cells.
The contribution of 8 anions at the corners to one unit cell is:
In the unit cell, one anion and one cation are present.
The mass of an anion is
and that of a cation is
The relation between
and
is as:
Convert the mass to grams as follows:
Similarly,
Thus, the mass of the unit cell will be:
The formula to calculate the edge length is as:
Substitute
for
and
for
in the above expression as:
Convert the edge length to
as follows:
The expression to calculate volume is as:
Substitute
in the above expression as:
Now, finally calculate density as:
Substitute
for
for
in the above expression as:
The density of the hypothetical ionic compound is
Want to see more full solutions like this?
Chapter 11 Solutions
EBK CHEMISTRY
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
- Please explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





