
Interpretation: The reagents used to accomplish the given transformations should be identified.
Concept Introduction:
Addition Reaction: It is defined as
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed via E1 (the reaction depends only on the substrate involved in the reaction) or E2 (the reaction depends on both of the substituents in the reaction) mechanism.
Substitution Reaction: The reaction in which one group gets substituted by other group. The types of substitution reactions are electrophilic and nucleophilic substitution reactions.
Anti-Markovnikov’s Addition Rule: The unsymmetrical
To identify: The reagents used to complete the given transformations.
Answer to Problem 11PP
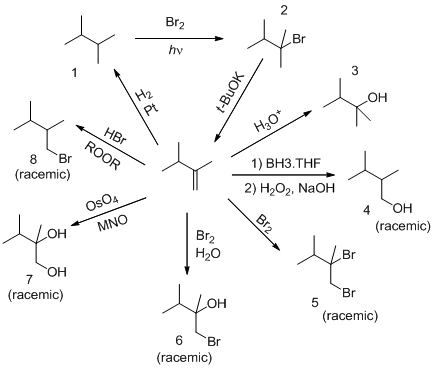
Explanation of Solution
Identify the reagents used in formation of products 1,2 and 3 from given substrate.
Analyzing the given product (1) shows that it lacks carbon-carbon double bond with respect to its reactant. Therefore, the given substrate undergoes reduction in presence of reagents
The given product 2 contains one extra bromine atom when comparing with 1 since product 1 undergoes halogenation reaction. Hence the suitable reagent used in this transformation is
In presence of tertiary butoxide the bromine group in product 2, gets eliminated and the tertiary butoxide abstracts protons from one carbon which finally results in the formation of given substrate.
Then examining product 3 shows that the carbon in carbon-carbon double bond in given substrate is protonated to give more stable carbocation and finally the nucleophile
Identify the reagents used in formation of products 4,5 and 6 from given substrate.
Examining product 4 shows that the substrate should undergoes anti-markovnikov addition of
Analyzing the product 5 clearly shows that it contains two bromine atoms extra which is substituted in the carbon-carbon double bond in given substrate which shows that suitable reagent for the transformation is
The product 6 shows that it contains one bromine atom and one
Identify the reagents used in formation of products of 7 and 8 from the given substrate.
Going through the product shows that it contains two hydroxyl groups hence suitable reagent for this transformation is
Product 8 shows that it contains bromine atom in less substituted atom which shows that anti-markovnikov addition happens hence suitable reagent for that is
The reagents used in the given transformations are identified by using the reaction in which the given substrate undergoes
Want to see more full solutions like this?
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
- Please help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forward
- Including activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forwardIncluding activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forward
- Can I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forward
- Can I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





