
Concept explainers
(a)
Interpretation:
The product of the reaction of dibutyl sulfide with
Concept introduction:
Hydrogen perioxide
Sulfur containing organic compounds has many similar chemical properties as oxygen contain compound such as ether and aclcohol.
Answer to Problem 11.45AP
The product of the reaction of dibutyl sulfide with

Explanation of Solution
The dibutyl sulfide undergoes an oxidation reaction with hydrogen peroxide. The dibutyl sulfide reacts with
The corresponding

Figure 1
The product of the reaction of dibutyl sulfide with
(b)
Interpretation:
The product of the reaction of dibutyl sulfide with
Concept introduction:
Hydrogen perioxide
Sulfur containing organic compounds has many similar chemical properties as oxygen contain compound such as ether and aclcohol.
Answer to Problem 11.45AP
The product of the reaction of dibutyl sulfide with
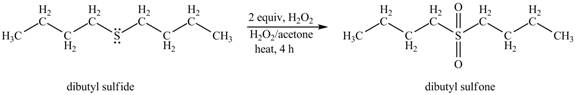
Explanation of Solution
The dibutyl sulfide undergoes an oxidation reaction with hydrogen peroxide. The dibutyl sulfide reacts with
The corresponding chemical reaction is shown below.
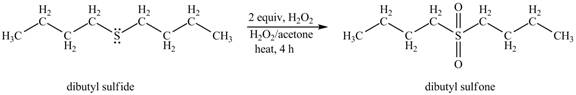
Figure 2
The product of the reaction of dibutyl sulfide with
(c)
Interpretation:
The product of the reaction of
Concept introduction:
The magnesium monoperoxyphthalate
Answer to Problem 11.45AP
The product of the reaction of
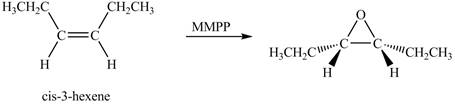
Explanation of Solution
The compound
The corresponding chemical reaction is shown below.
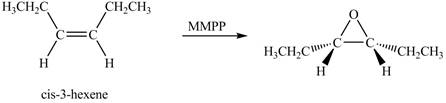
Figure 3
The product of the reaction of
(d)
Interpretation:
The product of the reaction of given compound with
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.45AP
The product of the reaction of given compound with
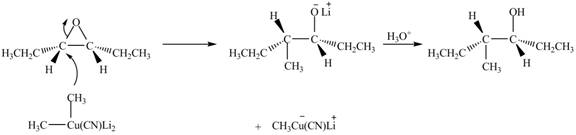
Explanation of Solution
The epoxide undergoes ring-opening reaction in the presence of acid. The dilithium dimethylcyanocuprate molecule generates a nucleophile
Figure 4
The product of the reaction of given compound with
(e)
Interpretation:
The product of the reaction of given compound with solvent
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.45AP
The product of the reaction of given compound with solvent
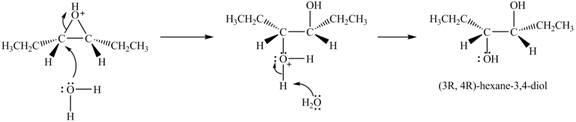
Explanation of Solution
The epoxide undergoes ring opening reaction in the presence of acid. The water molecule acts a nucleophile and attacks on the more substituted carbon atom of the epoxide ring to from

Figure 5
The product of the reaction of given compound with solvent
(f)
Interpretation:
The product of the reaction of given compound with periodic acid is to be predicated.
Concept introduction:
The periodic acid acts as a strong oxidizing agent. The periodic acid reacts with a vicinal diol to form two
Answer to Problem 11.45AP
The product of the reaction of given compound with periodic acid is shown below.
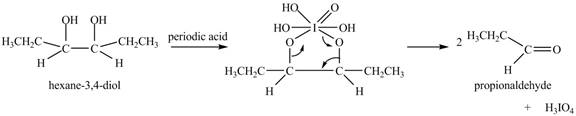
Explanation of Solution
The given compound is vicinal diol. It reacts with periodic acid to form two aldehydes. The carbon-carbon bond between the carbon atoms attached to two adjacent hydroxyl groups gets breaks. The corresponding chemical reaction is shown below.

Figure 6
The product of the reaction of given compound with periodic acid is shown in Figure 6.
(g)
Interpretation:
The product of the reaction of given compound with
Concept introduction:
The metal hydride reagents are good reducing agents such as
Answer to Problem 11.45AP
The product of the reaction of given compound with
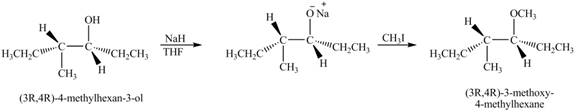
Explanation of Solution
The base
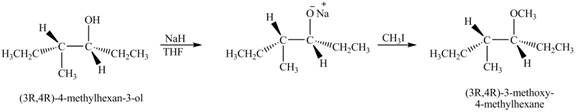
Figure 7
The product of the reaction of given compound with
(h)
Interpretation:
The product of the reaction of
Concept introduction:
Oxymercuration reaction is a type of reaction in which an alkene get converted into an alcohol. The mercuric acetate is used in the reaction as reagent. This reagent attacks the alkene to form a cyclic intermediate compound which further undergoes reduction to form alcohol.
Answer to Problem 11.45AP
The product of the reaction of
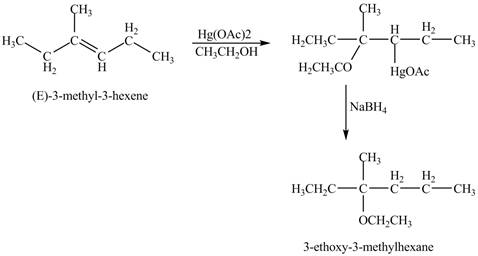
Explanation of Solution
The mercuric acetate
The corresponding chemical reaction is shown below.
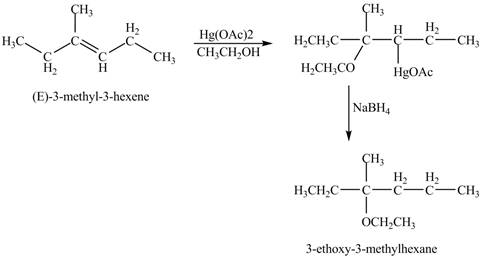
Figure 8
The product of the reaction of
(i)
Interpretation:
The product of the reaction of the given compound with acidic methanol is to be predicated.
Concept introduction:
The replacement or substitution of one
Answer to Problem 11.45AP
The product of the reaction of the given compound with acidic methanol is shown below.
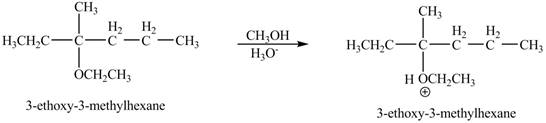
Explanation of Solution
The proton of the acid will protionate the ether. The protonated ether will increase the electrophilic characters of carbon atom attached to the oxygen atom. The methonal can acts as nuclephile, however the nucleophilic charater of methoxy-group and ethoxy group is similar. Therefore, no further reaction will take place.
The corresponding chemical reaction is shown below.
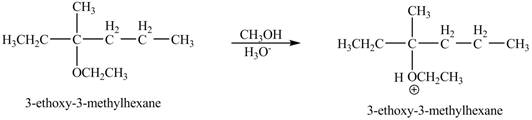
Figure 9
The product of the reaction of the given compound with acidic methanol is shown in Figure 9.
(j)
Interpretation:
The product of the reaction of
Concept introduction:
Grignard reagents are
Answer to Problem 11.45AP
The product of the reaction of
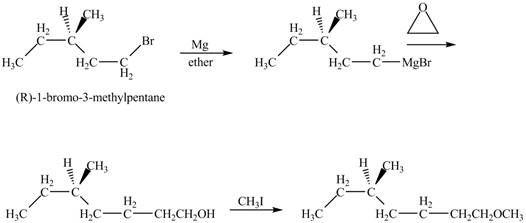
Explanation of Solution
The alkyl halide will react with magnisum metal to from gridnard reagent. These Grignard reagent reacts with epoixde to form alcohol. The alcohol reacts with
The corresponding chemical reaction is shown below.
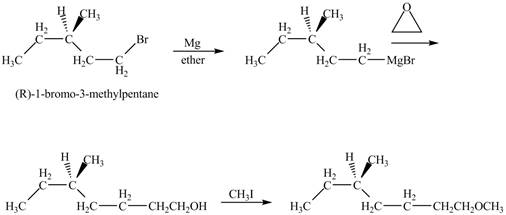
Figure 10
The product of the reaction of
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





