
Chemistry: Structure and Properties Plus Mastering Chemistry with Pearson eText -- Access Card Package (2nd Edition) (New Chemistry Titles from Niva Tro)
2nd Edition
ISBN: 9780134436524
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 110E
Interpretation Introduction
Interpretation:
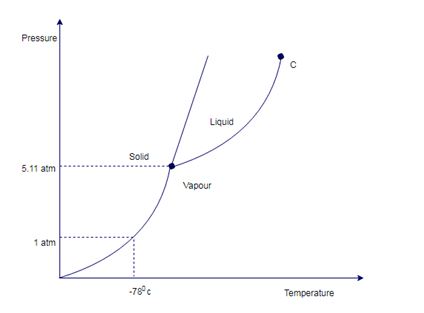
Sketch the phase diagram of carbon dioxide. It may or may not be possible to turn it into liquid by cooling it down at 1.0 atm and 25° C.
Concept introduction:
A pure substance generally has three phases at different condition. Representation of all the phases at different condition form phase diagram.
Phase diagram for carbon dioxide
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(2 pts) Why is O2 more stable as a diatomic molecule than S2?
Draw the Lewis structure for the polyatomic phosphite (PO¾³¯) a
anion. Be sure to include all resonance structures that satisfy the octet rule.
C
I A
[ ]¯
Decide whether these proposed Lewis structures are reasonable.
proposed Lewis structure
Is the proposed Lewis structure reasonable?
Yes.
:0:
Cl C C1:
0=0:
: 0 :
: 0 :
H C N
No, it has the wrong number of valence electrons.
The correct number is: ☐
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:* ☐
Yes.
No, it has the wrong number of valence electrons.
The correct number is: ☐
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
Yes.
☐
No, it has the wrong number of valence electrons.
The correct number is: ☐
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:* |
* If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many
times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".
Chapter 11 Solutions
Chemistry: Structure and Properties Plus Mastering Chemistry with Pearson eText -- Access Card Package (2nd Edition) (New Chemistry Titles from Niva Tro)
Ch. 11 - Why do ethanol and dimethyl ether have such...Ch. 11 - Why are intermolecular forces important?Ch. 11 - Prob. 3ECh. 11 - Prob. 4ECh. 11 - Prob. 5ECh. 11 - Which factors cause transitions between the solid...Ch. 11 - Describe the relationship between the state of a...Ch. 11 - Prob. 8ECh. 11 - Prob. 9ECh. 11 - Prob. 10E
Ch. 11 - Prob. 11ECh. 11 - Prob. 12ECh. 11 - Prob. 13ECh. 11 - What is the ion-dipole force? Why is it important?Ch. 11 - Prob. 15ECh. 11 - Prob. 16ECh. 11 - What is capillary action? How does it depend on...Ch. 11 - Explain what happens during the processes of...Ch. 11 - Why is vaporization endothermic? Why is...Ch. 11 - Prob. 20ECh. 11 - What is the heat of vaporization for a liquid, and...Ch. 11 - Explain the process of dynamic equilibrium. How is...Ch. 11 - What happens to a system in dynamic equilibrium...Ch. 11 - Prob. 24ECh. 11 - Prob. 25ECh. 11 - Prob. 26ECh. 11 - Prob. 27ECh. 11 - Prob. 28ECh. 11 - Prob. 29ECh. 11 - Prob. 30ECh. 11 - Prob. 31ECh. 11 - Examine the heating curve for water in section...Ch. 11 - What is a phase diagram? What is the significance...Ch. 11 - Draw a generic phase diagram and label its...Ch. 11 - Prob. 35ECh. 11 - Determine the kinds of intermolecular forces that...Ch. 11 - Determine the kinds of intermolecular forces that...Ch. 11 - Prob. 38ECh. 11 - Arrange these compounds in order of increasing...Ch. 11 - Prob. 40ECh. 11 - Pick the compound with the highest boiling point...Ch. 11 - Pick the compound with the highest boiling point...Ch. 11 - Prob. 43ECh. 11 - Prob. 44ECh. 11 - Prob. 45ECh. 11 - Prob. 46ECh. 11 - Prob. 47ECh. 11 - Water (a) “wets” some surfaces and beads up on...Ch. 11 - The structures of two isomers of heptanes are...Ch. 11 - Prob. 50ECh. 11 - Water in a glass tube that contains grease or oil...Ch. 11 - When a thin glass tube is put into water, the...Ch. 11 - Which evaporates more quickly: 55 mL of water in a...Ch. 11 - Prob. 54ECh. 11 - Spilling room temperature water over your skin on...Ch. 11 - Prob. 56ECh. 11 - The human body obtains 915 kJ of energy from a...Ch. 11 - Prob. 58ECh. 11 - Suppose that 0.95 g of water condenses on a 75.0 g...Ch. 11 - Prob. 60ECh. 11 - Prob. 61ECh. 11 - Prob. 62ECh. 11 - Prob. 63ECh. 11 - Prob. 64ECh. 11 - How much energy is released when 65.8 g of water...Ch. 11 - Prob. 66ECh. 11 - An 8.5 g ice cube is placed into 255 g of water....Ch. 11 - Prob. 68ECh. 11 - Prob. 69ECh. 11 - Prob. 70ECh. 11 - Prob. 71ECh. 11 - Prob. 72ECh. 11 - Prob. 73ECh. 11 - Prob. 74ECh. 11 - Prob. 75ECh. 11 - The high-pressure phase diagram of ice is shown...Ch. 11 - Prob. 77ECh. 11 - Prob. 78ECh. 11 - Prob. 79ECh. 11 - How is the density of solid water compared to that...Ch. 11 - Prob. 81ECh. 11 - Prob. 82ECh. 11 - Prob. 83ECh. 11 - Prob. 84ECh. 11 - Four ice cubes at exactly 00C with a total mass of...Ch. 11 - Prob. 86ECh. 11 - Draw a heating curve (such as the one in Figure...Ch. 11 - Draw a heating curve (such as the one in Figure...Ch. 11 - Prob. 89ECh. 11 - A sealed flask contains 0.55 g of water at 280C....Ch. 11 - Prob. 91ECh. 11 - Prob. 92ECh. 11 - Prob. 93ECh. 11 - Given that the heat of fusion of water is —6.02...Ch. 11 - The heat of combustion of CH4 is 890.4 kJ/mol, and...Ch. 11 - Prob. 96ECh. 11 - Prob. 97ECh. 11 - Prob. 98ECh. 11 - Prob. 99ECh. 11 - Prob. 100ECh. 11 - Prob. 101ECh. 11 - Prob. 102ECh. 11 - Prob. 103ECh. 11 - Prob. 104ECh. 11 - Prob. 105ECh. 11 - A substance has a triple point at a temperature of...Ch. 11 - The boiling of three compounds are tabulated here....Ch. 11 - Prob. 108ECh. 11 - Based on the heating curve for water, does it take...Ch. 11 - Prob. 110ECh. 11 - Prob. 111ECh. 11 - Prob. 1SAQCh. 11 - Liquid nitrogen boils at 77 K. This image depicts...Ch. 11 - Taking intermolecular forces into account, which...Ch. 11 - What substance experiences dipole-dipole forces?...Ch. 11 - Prob. 5SAQCh. 11 - Prob. 6SAQCh. 11 - Determine the amount of heat (in kJ) required to...Ch. 11 - Prob. 8SAQCh. 11 - Prob. 9SAQCh. 11 - Prob. 10SAQCh. 11 - Prob. 11SAQCh. 11 - Determine which state this substance is in at 1...Ch. 11 - Prob. 13SAQ
Knowledge Booster
Similar questions
- Draw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forwardPlease draw, not just describe!arrow_forward
- can you draw each step on a piece of a paper please this is very confusing to mearrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward
- > For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forwardHow to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forward
- Draw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning