
Consider a combined gas–steam power plant that has a net power output of 280 MW. The pressure ratio of the gas-turbine cycle is 11. Air enters the compressor at 300 K and the turbine at 1100 K. The combustion gases leaving the gas turbine are used to heat the steam at 5 MPa to 350°C in a heat exchanger. The combustion gases leave the heat exchanger at 420 K. An open feedwater heater incorporated with the steam cycle operates at a pressure of 0.8 MPa. The condenser pressure is 10 kPa. Assuming isentropic efficiencies of 100 percent for the pump, 82 percent for the compressor, and 86 percent for the gas and steam turbines, determine (a) the mass flow rate ratio of air to steam, (b) the required rate of heat input in the combustion chamber, and (c) the thermal efficiency of the combined cycle.
(a)
The mass flow rate ratio of the air to the steam.
Answer to Problem 86P
The mass flow rate ratio of the air to the steam is
Explanation of Solution
Show the
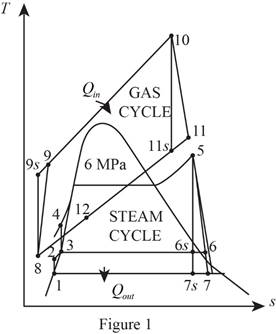
Refer Figure 1.
Consider the gas cycle (topping cycle) and their respective process states such as 8, 9,
At state 8:
The air enters the compressor at the temperature of
Refer Table A-17, “Ideal-gas properties of air”.
The enthalpy
Write the relative pressure and absolute pressure relation for the process 8-9-
Here, the relative pressure is
Write the formula for isentropic efficiency of compressor for the process 8-9-
Here, the enthalpy is
At state 10:
The air enters the turbine at the temperature of
Refer Table A-17, “Ideal-gas properties of air”.
The enthalpy
Write the relative pressure and absolute pressure relation for the process 10-11-
Write the formula for isentropic efficiency of gas turbine
At state 12: (heat exchanger)
The enthalpy
Refer Figure 1.
Consider the steam cycle (bottoming cycle) and their respective process states such as 1, 2, 3, 4, 5, 6,
At state 1: (Pump I inlet)
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 2:
Write the formula for work done by the pump during process 1-2.
Here, the specific volume is
Write the formula for enthalpy
At state 3: (Pump II inlet)
The water exits the open feed water heater as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 4:
Write the formula for work done by the pump during process 3-4.
Here, the specific volume is
Write the formula for enthalpy
At state 5:
The steam enters the turbine as superheated vapour.
Refer Table A-6, “Superheated water”.
The enthalpy
At state
The steam expanded to the pressure of
The quality of water at state
The enthalpy at state
Here, the enthalpy is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
The isentropic efficiency of the steam turbine for the process 5-6-
At state
The steam enters the condenser at the pressure of
The quality of water at state
The enthalpy at state
Here, the subscript
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
The isentropic efficiency of the steam turbine for the process 5-7-
Here, the subscript
Write the general energy rate balance equation.
Here, the rate of energy in is
Consider the heat exchanger operates on steady state. Hence, the rate of change in net energy of the system is zero.
The Equation (XVI) is reduced as follows for the heat exchanger.
Here, the mass flow rate of air is
Conclusion:
Substitute
Refer Table A-17, “Ideal-gas properties of air”.
The enthalpy
Substitute
Substitute
Refer Table A-17, “Ideal-gas properties of air”.
The enthalpy
Substitute
Substitute
Substitute
Substitute
Equation (VII).
Substitute
From Figure 1.
Substitute
Substitute
Equation (X).
Substitute
Substitute
Substitute
Equation (XIII).
Substitute
Substitute
Thus, the mass flow rate ratio of the air to the steam is
(b)
The required rate of heat input in the combustion chamber.
Answer to Problem 86P
The required rate of heat input in the combustion chamber is
Explanation of Solution
Refer Equation (XV).
Consider the open feed water heater operates on steady state. Hence, the rate of change in net energy of the system is zero.
Write the energy rate balance equation for open feed water heater.
Rewrite the Equation (XVII) in terms of mass fraction
Here, the mass fraction steam extracted from the turbine to the inlet mass of the boiler
Write the formula for work output of the steam turbine.
Write the formula for net work output of the steam cycle.
Write the formula for net work output of the gas cycle.
Write the formula for the net work output of the gas-steam cycle per unit mass of gas.
Write the formula for mass flow rate of air through the compressor.
Write the formula for rate of heat input to the combustion chamber.
Conclusion:
Substitute
Equation (XVIII).
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Equation (XXIV).
Thus, the required rate of heat input in the combustion chamber is
(c)
The thermal efficiency of the combined cycle.
Answer to Problem 86P
The thermal efficiency of the combined cycle is
Explanation of Solution
Write the formula for thermal efficiency.
Conclusion:
Substitute
Thus, the thermal efficiency of the combined cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
CENGEL'S 9TH EDITION OF THERMODYNAMICS:
- CORRECT ANSWER AND COMPLETE FBD ONLY. I PREFER HANDWRITTEN BUT ITS OKAY IF NOT. I WILL UPVOTE 3. The frame has pin supports at A and E, subject to a wind load. Treat joint C to be an internal hinge. Given:Dimensions, H1 = 3.0 m; H2 = 4.5 m; L = 10.0 mWind loads, wWL (AB) = 4.8 kN/m; wWL (BC) = 3.9 kN/m; wWL (CD) = 1.5 kN/m; wWL (DE) = 1.2 kN/mMembers are made of A36 steel Wide Flange Section with the following properties:Area, A = 64000 mm2Depth, d = 762 mmFlange width, bf = 371 mmThickness of web, tw = 32 mmThickness of flange, tf = 57.9 mmMoment of inertia about x-axis, Ix = 6080 x 106 mm4The wide flange is oriented so that the bending is about the x-axis1. Calculate the stress in member AB, due to the axial load it carries, indicate if tension or compression.Answer: 0.0476 MPa Tension2. Calculate the stress in member DE, due to the axial load it carries, indicate if tension or compression.Answer: 0.2351 MPa Compression3. Calculate the maximum bending stress at B. Answer: 4.282 MPaarrow_forward32 mm 32 mm b' c' C 32 mm 32 mm b PROBLEM 6.41 a The extruded beam shown has a uniform wall thickness of 3 mm. Knowing that the vertical shear in the beam is 9 kN, determine the shearing stress at each of the five points indicated.arrow_forwardIn a structural reliability problem, the resistance (capacity) R and load effect (demand) S random variables associated with a failure mode of the structure of interest are normally distributed and statistically independent with the following probability distribution parameters (or statistics) in consistent units: MR = 12, σR = 3 μs = 5, σs = 2 (a) Determine the exact probability of failure pF ·arrow_forward
- The resistance R and load effect S for a given failure mode are statistically independent random variables with marginal PDF's 1 fR (r) = 0≤r≤100 100' fs(s)=0.05e-0.05s (a) Determine the probability of failure by computing the probability content of the failure domain defined as {rarrow_forwardPlease solve this problem as soon as possible My ID# 016948724arrow_forwardThe gears shown in the figure have a diametral pitch of 2 teeth per inch and a 20° pressure angle. The pinion rotates at 1800 rev/min clockwise and transmits 200 hp through the idler pair to gear 5 on shaft c. What forces do gears 3 and 4 transmit to the idler shaft? TS I y 18T 32T This a 12 x 18T C 48T 5arrow_forwardQuestion 1. Draw 3 teeth for the following pinion and gear respectively. The teeth should be drawn near the pressure line so that the teeth from the pinion should mesh those of the gear. Drawing scale (1:1). Either a precise hand drawing or CAD drawing is acceptable. Draw all the trajectories of the involute lines and the circles. Specification: 18tooth pinion and 30tooth gear. Diameter pitch=P=6 teeth /inch. Pressure angle:20°, 1/P for addendum (a) and 1.25/P for dedendum (b). For fillet, c=b-a.arrow_forward5. The figure shows a gear train. There is no friction at the bearings except for the gear tooth forces. The material of the milled gears is steel having a Brinell hardness of 170. The input shaft speed (n2) is 800 rpm. The face width and the contact angle for all gears are 1 in and 20° respectively. In this gear set, the endurance limit (Se) is 15 kpsi and nd (design factor) is 2. (a) Find the revolution speed of gear 5. (b) Determine whether each gear satisfies the design factor of 2.0 for bending fatigue. (c) Determine whether each gear satisfies the design factor of 2.0 for surface fatigue (contact stress). (d) According to the computation results of the questions (b) and (c), explain the possible failure mechanisms for each gear. N4=28 800rpm N₁=43 N5=34 N₂=14 P(diameteral pitch)=8 for all gears Coupled to 2.5hp motorarrow_forward1. The rotating steel shaft is simply supported by bearings at points of B and C, and is driven by a spur gear at D, which has a 6-in pitch diameter. The force F from the drive gear acts at a pressure angle of 20°. The shaft transmits a torque to point A of TA =3000 lbĘ in. The shaft is machined from steel with Sy=60kpsi and Sut=80 kpsi. (1) Draw a shear force diagram and a bending moment diagram by F. According to your analysis, where is the point of interest to evaluate the safety factor among A, B, C, and D? Describe the reason. (Hint: To find F, the torque Tд is generated by the tangential force of F (i.e. Ftangential-Fcos20°) When n=2.5, K=1.8, and K₁ =1.3, determine the diameter of the shaft based on (2) static analysis using DE theory (note that fatigue stress concentration factors need to be used for this question because the loading condition is fatigue) and (3) a fatigue analysis using modified Goodman. Note) A standard diameter is not required for the questions. 10 in Darrow_forward3 N2=28 P(diametral pitch)=8 for all gears Coupled to 25 hp motor N3=34 Full depth spur gears with pressure angle=20° N₂=2000 rpm (1) Compute the circular pitch, the center-to-center distance, and base circle radii. (2) Draw the free body diagram of gear 3 and show all the forces and the torque. (3) In mounting gears, the center-to-center distance was reduced by 0.1 inch. Calculate the new values of center-to-center distance, pressure angle, base circle radii, and pitch circle diameters. (4)What is the new tangential and radial forces for gear 3? (5) Under the new center to center distance, is the contact ratio (mc) increasing or decreasing?arrow_forward2. A flat belt drive consists of two 4-ft diameter cast-iron pulleys spaced 16 ft apart. A power of 60 hp is transmitted by a pulley whose speed is 380 rev/min. Use a service factor (Ks) pf 1.1 and a design factor 1.0. The width of the polyamide A-3 belt is 6 in. Use CD=1. Answer the following questions. (1) What is the total length of the belt according to the given geometry? (2) Find the centrifugal force (Fc) applied to the belt. (3) What is the transmitted torque through the pulley system given 60hp? (4) Using the allowable tension, find the force (F₁) on the tight side. What is the tension at the loose side (F2) and the initial tension (F.)? (5) Using the forces, estimate the developed friction coefficient (f) (6) Based on the forces and the given rotational speed, rate the pulley set. In other words, what is the horse power that can be transmitted by the pulley system? (7) To reduce the applied tension on the tight side, the friction coefficient is increased to 0.75. Find out the…arrow_forwardThe tooth numbers for the gear train illustrated are N₂ = 24, N3 = 18, №4 = 30, №6 = 36, and N₁ = 54. Gear 7 is fixed. If shaft b is turned through 5 revolutions, how many turns will shaft a make? a 5 [6] barrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning

