
Interpretation:
Major product has to be predicted for the given reaction.
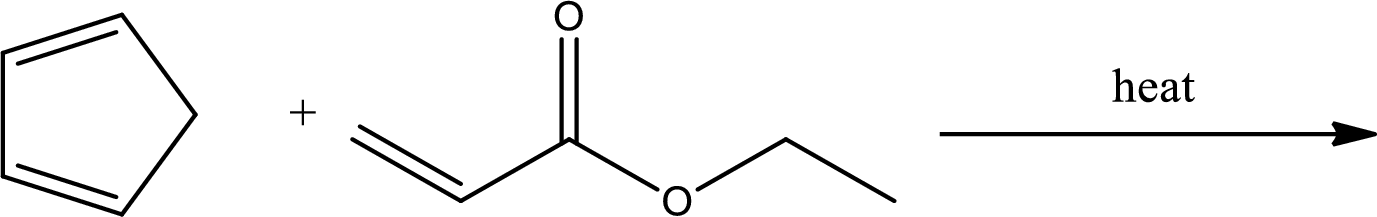
Concept Introduction:
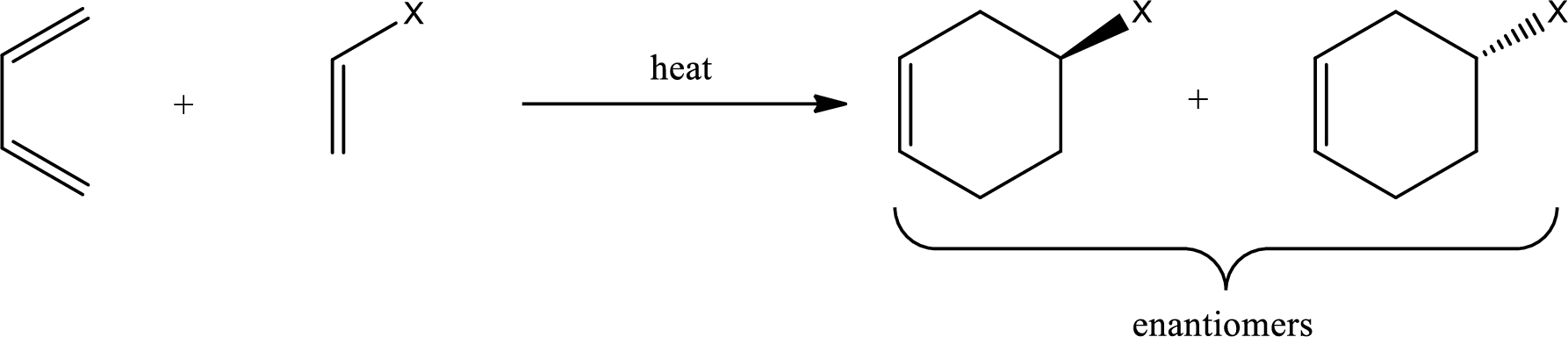
Diels-Alder reaction is the 4+2 addition reaction. Here a total of six pi bonds are involved resulting in formation of one pi bond and two single bonds. Diels-Alder reaction occurs via a single concerted step and not by involvement of ions. If a monosubstituted ethylene is used, the product obtained contains a stereocenter. This can be represented as,
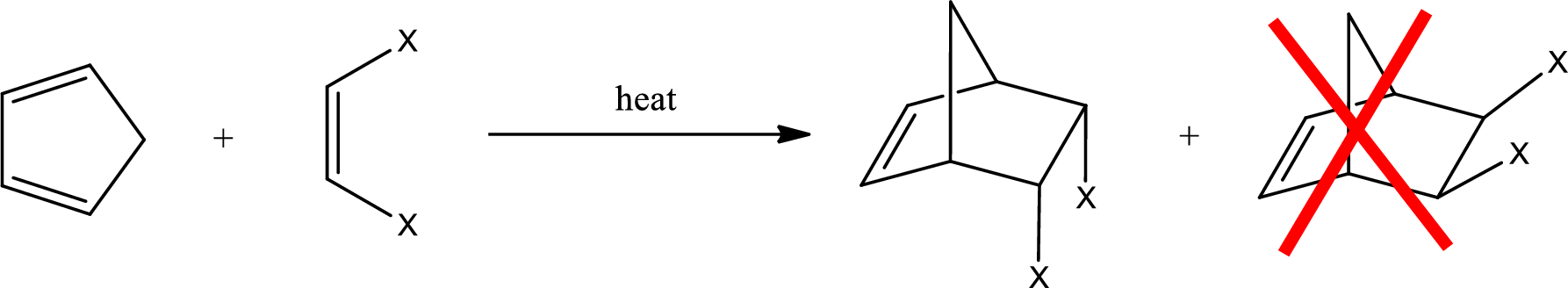
If a cyclic diene is considered, then the product obtained will be a bicyclic compound. Diels-Alder reaction that involves cyclopentadiene results in a bicyclic structure. If the dienophile has a cis configuration, then the two groups have to be in cis configuration to each other. The product obtained will have the groups in endo position. It is a meso compound.
If the dienophile has a trans configuration, then the two groups have to be in trans configuration to each other. The product obtained will have the groups as shown below,
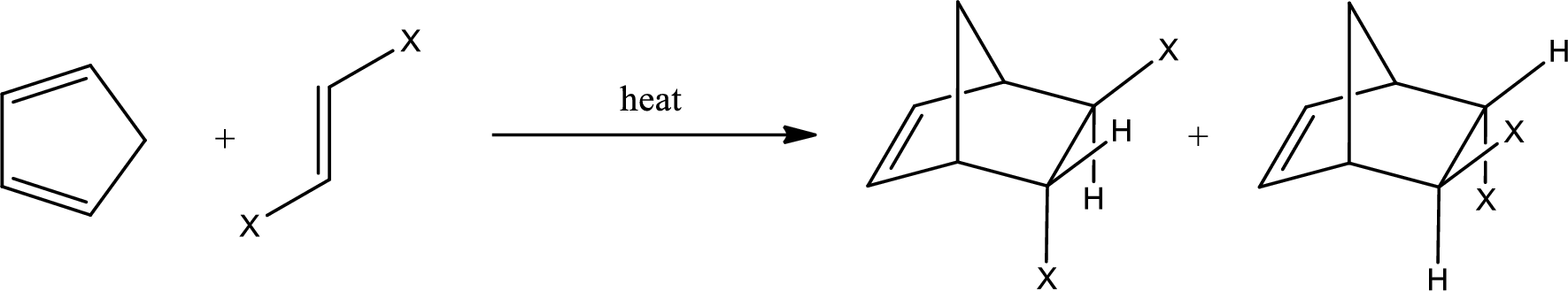
The above two structures are enantiomers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
- Synthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forwardShown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forward
- Draw a reasonable mechanism for the following reaction:arrow_forwardDraw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

