
MODERN PHYSICS (LOOSELEAF)
4th Edition
ISBN: 9781119495550
Author: Krane
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 4Q
How would Figure 10.13 change if the temperature of the gas were increased?
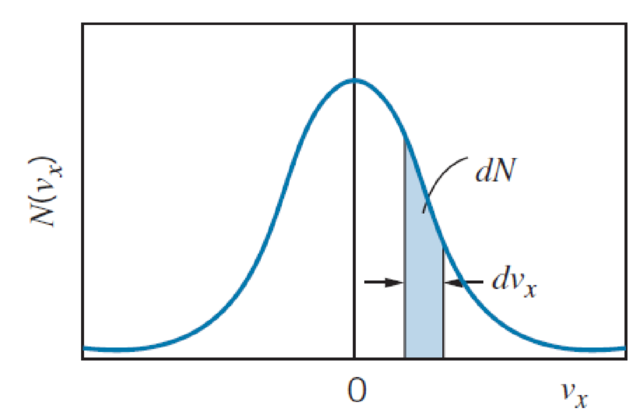
FIGURE 10.13 The Maxwell velocity distribution for gas molecules. The distribution is centered on vx = 0. The shaded strip represents the number of molecules with velocity components between vx and vx + dvx.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a) Consider the following function, where A is a constant.
y(x,t) = A(x — vt).
Can this represent a wave that travels along? Explain.
b) Which of the following are possible traveling waves, provide your reasoning and give the velocity of the
wave if it can be a traveling wave.
e-(a²x²+b²²-2abtx
b.1) y(x,t)
b.2) y(x,t)
=
=
A sin(ax² - bt²).
2
b.3) y(x,t)
=
A sin 2π
(+)
b.4) y(x,t)
=
A cos² 2π(t-x).
b.5) y(x,t)
=
A cos wt sin(kx - wt)
The capacitor in (Figure 1) is initially uncharged. The switch is closed at t=0. Immediately after the switch is closed, what is the current through the resistor R1, R2, and R3? What is the final charge on the capacitor? Please explain all steps.
Suppose you have a lens system that is to be used primarily for 620-nm light. What is the second thinnest coating of fluorite (calcium fluoride) that would be non-reflective for this wavelength?
× nm
434
Chapter 10 Solutions
MODERN PHYSICS (LOOSELEAF)
Additional Science Textbook Solutions
Find more solutions based on key concepts
83. Calculate the freezing point and boiling point each aqueous solution, assuming complete dissociation of the...
Chemistry: Structure and Properties (2nd Edition)
11.57 Draw the cis and trans isomers for each of the following: (11.6)
a. 2-pentene
b. 3-hexene
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
Which one of the following is not a fuel produced by microorganisms? a. algal oil b. ethanol c. hydrogen d. met...
Microbiology: An Introduction
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
In general, how do surface temperatures vary from the equator toward the poles? What is the likely cause of thi...
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The angle between the axes of two polarizing filters is 19.0°. By how much does the second filter reduce the intensity of the light coming through the first? I = 0.106 40 xarrow_forwardAn oil slick on water is 82.3 nm thick and illuminated by white light incident perpendicular to its surface. What color does the oil appear (what is the most constructively reflected wavelength, in nanometers), given its index of refraction is 1.43? (Assume the index of refraction of water is 1.33.) wavelength color 675 × nm red (1 660 nm)arrow_forwardA 1.50 μF capacitor is charging through a 16.0 Ω resistor using a 15.0 V battery. What will be the current when the capacitor has acquired 1/4 of its maximum charge? Please explain all stepsarrow_forward
- In the circuit shown in the figure (Figure 1), the 6.0 Ω resistor is consuming energy at a rate of 24 J/s when the current through it flows as shown. What are the polarity and emf of the battery E, assuming it has negligible internal resistance? Please explain all steps. I know you need to use the loop rule, but I keep getting the answer wrong.arrow_forwardIf you connect a 1.8 F and a 2.6 F capacitor in series, what will be the equivalent capacitance?arrow_forwardSuppose that a particular heart defibrillator uses a 1.5 x 10-5 Farad capacitor. If it is charged up to a voltage of 7300 volts, how much energy is stored in the capacitor? Give your answer as the number of Joules.arrow_forward
- The voltage difference across an 8.3 nanometer thick cell membrane is 6.5 x 10-5volts. What is the magnitude of the electric field inside this cell membrane? (Assume the field is uniform, and give your answer as the number of Volts per meter... which is the same as the number of Newtons per Coulomb.)arrow_forwardThree identical capacitors are connected in parallel. When this parallel assembly of capacitors is connected to a 12 volt battery, a total of 3.1 x 10-5 coulombs flows through the battery. What is the capacitance of one individual capacitor? (Give your answer as the number of Farads.)arrow_forwardSuppose you construct your own capacitor by placing two parallel plates at a distance 0.27 meters apart. The plates each have a surface area of 0.64 square meters. What is the capacitance of this setup? (Give your answer as the number of Farads.)arrow_forward
- Draw a diagram with the new arrows. No they do not point all towards the center.arrow_forwardExample In Canada, the Earth has B = 0.5 mŢ, pointing north, 70.0° below the horizontal. a) Find the magnetic force on an oxygen ion (O2) moving due east at 250 m/s b) Compare the |FB| to |FE| due to Earth's fair- weather electric field (150 V/m downward).arrow_forwardFour charges, qa, qb, qa, and qd are fixed at the corners of a square. A charge q that is free to move located at the exact center of the square. Classify the scenarios described according to the force that would be exerted on the center charge q. Assume in each case that q is a positive charge. Do not assume that the fixed charges have equal magnitudes unless the scenario defines such an equality. qa Яс q %b Force is zero Force is to the left Force is to the right Force is undeterminedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY