
a)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
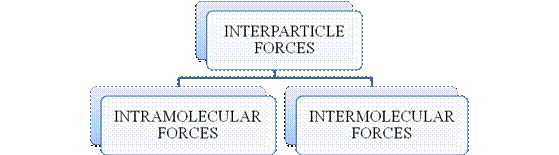
Figure 1
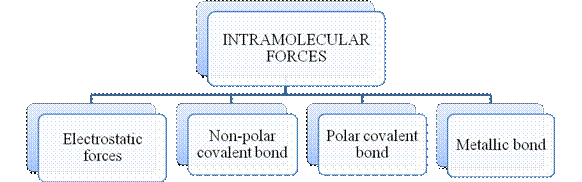
Figure 2
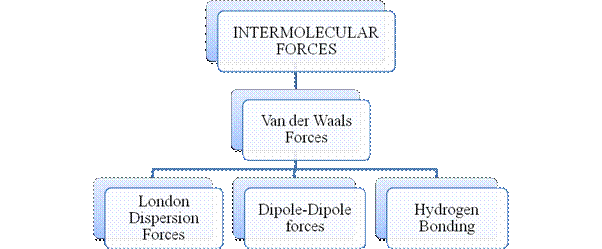
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
b)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
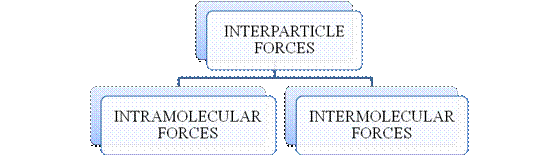
Figure 1
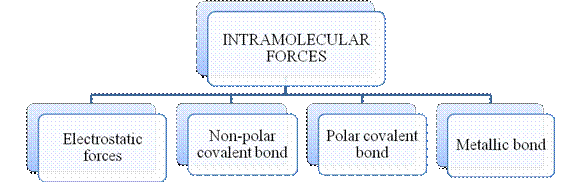
Figure 2
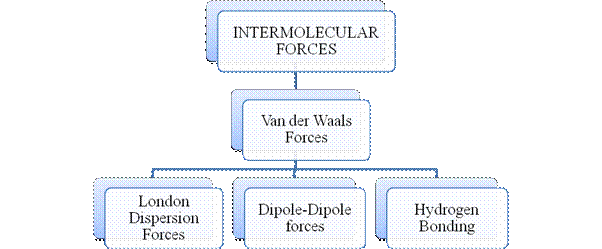
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
c)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
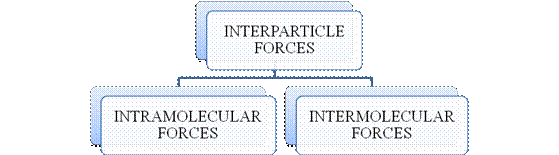
Figure 1

Figure 2
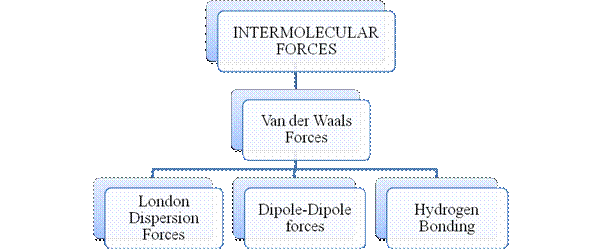
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
d)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
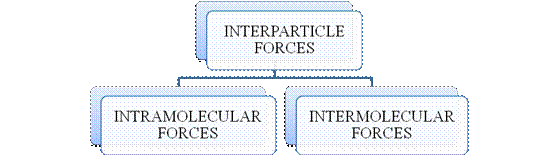
Figure 1

Figure 2

Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
Trending nowThis is a popular solution!

Chapter 10 Solutions
EBK CHEMISTRY
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward
- Write the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forwardClick the "draw structure" button to launch the drawing utility. Draw the structure of the alkene that yields the following set of oxidative cleavage products? draw structure ...arrow_forwardWrite the mechanism for the reaction.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





