
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260826791
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 37P
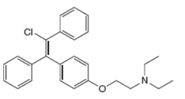
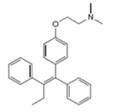
Label the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.
At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?
Please predict the products for each of the
following reactions.
Clearly show the regiochemistry (Markovnikov
vs anti-Markovnikov) and stereochemistry
(syn- vs anti- or both).
If a mixture of enantiomers is formed, please
draw all the enantiomers.
Hint: In this case you must choose the best
answer to demonstrate the stereochemistry of
H2 addition.
1.03
2. (CH3)2S
BIZ
CH₂OH
2. DMS
KMnO4, NaOH
ΖΗ
Pd or Pt (catalyst)
HBr
20 1
HBr
ROOR (peroxide)
HO
H-SO
HC
12 11 10
BH, THE
2. H2O2, NaOH
Brz
cold
HI
19
18
17
16
MCPBA
15
14
13
A
Br
H₂O
BH3⚫THF
Brz
EtOH
Pd or Ni (catalyst)
D₂ (deuterium)
1. Os04
2. H2O2
CH3CO3H
(peroxyacid)
1. MCPBA
2. H₂O*
H
B
+
H
H
H
"H
C
H
H
D
Chapter 10 Solutions
ORGANIC CHEMISTRY
Ch. 10.1 - Prob. 1PCh. 10.2 - Problem 10.2 How many degrees of unsaturation are...Ch. 10.3 - Give the IUPAC name for each alkene. abcdeCh. 10.3 - Give the IUPAC name for each polyfunctional...Ch. 10.3 - Prob. 9PCh. 10.6 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10.7 - Prob. 12PCh. 10.9 - Problem 10.13 What product is formed when each...Ch. 10.9 - Prob. 14PCh. 10.10 - Problem 10.15 Draw the products formed when each...
Ch. 10.10 - Prob. 16PCh. 10.10 - Prob. 17PCh. 10.10 - Addition of HBr to which of the following alkenes...Ch. 10.11 - Problem 10.19 Draw the products, including...Ch. 10.11 - Prob. 20PCh. 10.12 - Problem 10.21 What two alkenes give rise to each...Ch. 10.12 - Prob. 22PCh. 10.13 - Problem 10.23 Draw the products of each reaction,...Ch. 10.14 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10.15 - Prob. 25PCh. 10.16 - Problem 10.26 What alkylborane is formed from...Ch. 10.16 - Draw the products formed when each alkene is...Ch. 10.16 - What alkene can be used to prepare each alcohol as...Ch. 10.16 - Prob. 29PCh. 10.17 - Draw the products of each reaction using the two...Ch. 10.18 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 34PCh. 10 - 10.35 Calculate the number of degrees of...Ch. 10 - Prob. 36PCh. 10 - Label the alkene in each drug as E or Z....Ch. 10 - Give the IUPAC name for each compound. a. c. e. b....Ch. 10 - Prob. 39PCh. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 41PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 43PCh. 10 - Prob. 44PCh. 10 - Prob. 45PCh. 10 - Draw the products formed when (CH3)2C=CH2 is...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 48PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 50PCh. 10 - Draw all stereoisomers formed in each reaction. a....Ch. 10 - Draw the products of each reaction, including...Ch. 10 - Prob. 53PCh. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 60PCh. 10 - Prob. 61PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - 10.65 Draw a synthesis of each compound from...
Additional Science Textbook Solutions
Find more solutions based on key concepts
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forwardExplain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forward
- Briefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forwardThermodynamic analysis of electrified interfaces.arrow_forward
- What is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY