
Concept explainers
(a)
Interpretation:
For the given reaction the under given conditions the valence electrons present in the given compound should be determined.
Concept Introduction:
Valence electrons: The outermost electrons present in the compound other than the inner core electrons are denoted as valence electrons. The valence electrons are responsible for the bond formation.
There are about 19 valence electrons present in the given compound.
(a)
Explanation of Solution
The given compound
Similarly the chlorine atom contains 7 valence electrons in its outer most shell which totally comprises of
(b)
Interpretation:
For the given reaction the under given conditions the valence electrons present in the given compound
Concept introduction:
Lewis dot structure: It is the representation of chemical substance that how the valence are represented as dot and placed around the each atom present in the substance by considering the number of atoms with their valence electrons and number of bonds present in the given chemical substance.
(b)
Answer to Problem 116SCQ
The electron dot structure for the given compound is as follows,
Explanation of Solution
The electron dot structure for the given
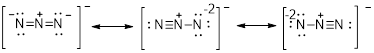
There are two oxygen atoms and one chlorine atom present in the given compound.
The oxygen atom contains 8 electrons that is represented as follows,
Similarly, the electrons present in the chlorine is represented as follows
Therefore, the chemical substance totally, has 20 valence electrons with it since addition of valence electrons present in the atom of the given compound says that. There are 2 O atoms each with 6 valence electrons one chlorine atom with 7 valence electrons and one negative charge which denotes present of one extra electrons which totally equals to 20.
The valence electrons are represented as dot around each atom present in the compound such that each atom tends to exhibit stable configuration that is 8electrons in its valence shell.
(c)
Interpretation:
For the given reaction the under given conditions the angle and the mass of
Concept introduction:
Hybridization refers to mixing of orbitals. The orbitals of different atoms overlap with each other and form a set of new orbitals called hybrid orbitals. The number of orbitals that are combined equal to the number of hybrid orbitals formed by combination.
An isolated atom remains in excited state and comes back to ground state. In this situation, it is difficult for orbitals to hybridize.
Hybridization is a hypothetical concept. It refers to mixing of atomic orbitals and the resultant orbitals formed are known as hybrid orbitals. After hybridization, the orbitals cannot be distinguished individually. Based on hybridization one can predict the geometry of the molecule though some deviations do exist.
(c)
Answer to Problem 116SCQ
The chlorine atom in the given compound is found to be
Explanation of Solution
The given compound contains chlorine with two oxygen atoms results to have two bonds with it.
The electronic configuration of Chlorine and oxygen is as follows,
Electronic configuration of chlorine is
The available orbitals that is s orbitals, one p orbital from chlorine and two p orbitals from oxygen atom gets hybridized and results to form new hybrid orbitals classified as
The central chlorine in compound
(d)
Interpretation:
For the given reaction the under given conditions the valence electrons present in the given compound
Concept introduction:
Bond angle: The angle formed between two bonds that is where two atoms gets bonded with the third central atom present.
(d)
Answer to Problem 116SCQ
The species
Explanation of Solution
The central chlorine in compound
Therefore,
(e)
Interpretation:
For the given reaction the under given conditions the mass of
Concept introduction:
Ideal gas equation:
Any gas is described by using four terms namely pressure, volume, temperature and the amount of gas. Thus combining three laws namely Boyle’s, Charles’s Law and Avogadro’s Hypothesis the following equation could be obtained. It is referred as ideal gas equation.
Under some conditions gases don not behave like ideal gas that is they deviate from their ideal gas properties. At lower temperature and at high pressures the gas tends to deviate and behave like real gases.
Boyle’s Law:
At given constant temperature conditions the mass of given ideal gas in inversely proportional to its volume.
Charles’s Law:
At given constant pressure conditions the volume of ideal gas is directly proportional to the absolute temperature.
Avogadro’s Hypothesis:
Two equal volumes of gases with same temperature and pressure conditions tend to have same number of molecules with it.
The relationship between partial pressure and
(e)
Answer to Problem 116SCQ
The mass of given compound formed is equal to
Explanation of Solution
Given:
First the given mass of
There are about
Then, the amount of
Now, analyzing the given chemical equation it is evident that 2 moles of
Similarly, there are
Analyzing the above calculations we have only about
Examining the given chemical reaction show that 1 mole of
Therefore, available amount of
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry and Chemical Reactivity - AP Edition
- Which element cannot interact with hydrogen through hydrogen bonds? Group of answer choices O S Br Narrow_forwardWhich of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forwardWhich of the following forms of hydrogen is the least stable? Group of answer choices H H2 H− H+arrow_forward
- Consider the following reduction half reactions and standard reduction potentials: Fe3+ + e− → Fe2+ Eo = +0.77 V Fe2+ + e− → Fe(s) Eo = -0.44 V Which of the following statements is true? Group of answer choices The Fe2+ reduction to Fe(s) is spontaneous. Fe2+ can disproportionate into Fe3+ and Fe(s) The Fe3+ reduction to Fe2+ is not spontaneous. Fe3+ and Fe(s) can undergo a comproportionation reaction to form Fe2+arrow_forwardAccording to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardConsider the redox reaction: 2 P4 + 8 OH− + 4 H2O → 4 PH3 + 4 HPO32− The element oxidized is ["", "", ""] , the element reduced is ["", "", ""] , one of the oxidizing agents is ["", "", ""] , and the reducing agent is ["", "", ""] .arrow_forward
- What is the missing reactant in this organic reaction? OH H + R Δ CH3-CH2-CH-CH3 O CH3 CH3-CH2-C-O-CH-CH2-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answe box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C O2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cerarrow_forwardPredict the product of this organic reaction: CH3 NH2 Δ CH3-CH-CH3 + HO-C-CH2-N-CH3 P+H₂O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. Xarrow_forwardIn the scope of the SCH4U course, please thoroughly go through the second questionarrow_forward
- Please help me solve these two problems. Thank you in advance.arrow_forwardNaming and drawing unsubstituted esters Write the systematic name of each organic molecule: Explanation structure Check name Х 2/5arrow_forwardPredict the product of this organic reaction: =0 CH3-O-CH2-C-OH + CH3-OH H P+H₂O A Specifically, in the drawing area below draw the condensed structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. ☐arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


