
Concept explainers
(a)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
The dipole moment arises when there is a separation of charges between two ions or atoms involved in the bond. The dipole moment is a vector quantity and its direction towards the most electronegative atom.
The direction of the dipole moment is represented as follows:
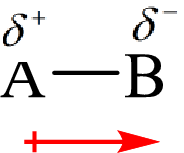
The polar and non-polar molecule can be identified on the basis of the net dipole moment. Polar molecules have non zero value of net dipole moment and the non polar molecules have zero net dipole moment.
(b)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge.
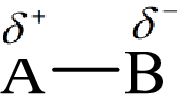
Here, B is the electronegative atom and A is the electropositive atom.
Bond polarity can be estimated by
Here, B is the electronegative atom and A is the electropositive atom.
The greater electronegativity difference between the atoms induces the greater polarity in the bond thus it generates higher dipole moment.
(c)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
The dipole moment arises when there is a separation of charges between two ions or atoms involved in the bond. The dipole moment is a vector quantity and its direction towards the most electronegative atom.
The direction of the dipole moment is represented as follows:
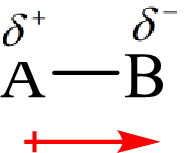
The polar and non-polar molecule can be identified on the basis of the net dipole moment. Polar molecules have non zero value of net dipole moment and the non polar molecules have zero net dipole moment.
(d)
Interpretation:
The molecule that has greater dipole moment among
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge.
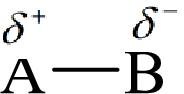
Here, B is the electronegative atom and A is the electropositive atom.
Bond polarity can be estimated by
Here, B is the electronegative atom and A is the electropositive atom.
The greater electronegativity difference between the atoms induces the greater polarity in the bond thus it generates higher dipole moment.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
CHEMISTRY:MOLECULAR...(LL) W/ALEKS
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





