
Concept explainers
(a)
Interpretation:
Line formula for given
(a)
Explanation of Solution
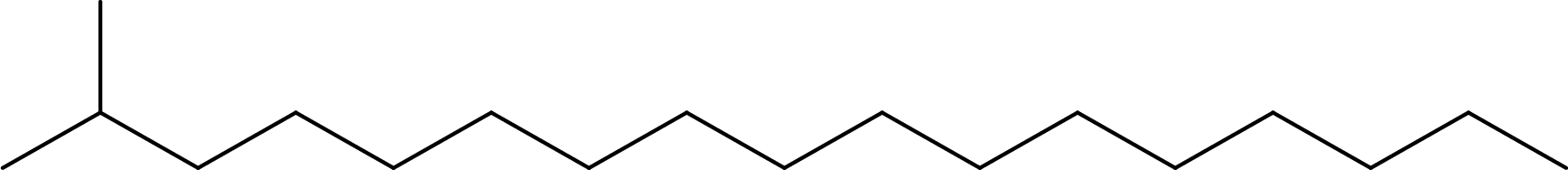
Line formula for 2-methylheptadecane:
From the given name, the parent alkane is found to be heptadecane. Substituent that is present is a methyl group on carbon-2. Line formula for 2-methylheptadecane can be drawn as,
Line formula for 17,21-dimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are two methyl groups on carbon-17 and carbon-21. Line formula for 17,21-dimethylheptatriacontane can be drawn as,

Line formula for 15,19-dimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are two methyl groups on carbon-15 and carbon-19. Line formula for 15,19-dimethylheptatriacontane can be drawn as,

Line formula for 15,19,23-trimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are three methyl groups on carbon-15, carbon-19 and carbon-23. Line formula for 15,19,23-trimethylheptatriacontane can be drawn as,

(c)
Interpretation:
Molar mass of given alkanes has to be calculated..
(c)
Explanation of Solution
Molar mass of 2-methylheptadecane:
Molecular formula of 2-methylheptadecane is given as
Molar mass of 2-methylheptadecane is calculated as shown below,
Therefore, molar mass of 2-methylheptadecane is
Molar mass of 17,21-dimethylheptatriacontane:
Molecular formula of 17,21-dimethylheptatriacontane is given as
Molar mass of 17,21-dimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 17,21-dimethylheptatriacontane is
Molar mass of 15,19-dimethylheptatriacontane:
Molecular formula of 15,19-dimethylheptatriacontane is given as
Molar mass of 15,19-dimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 15,19-dimethylheptatriacontane is
Molar mass of 15,19,23-trimethylheptatriacontane:
Molecular formula of 15,19,23-trimethylheptatriacontane is given as
Molar mass of 15,19,23-trimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 15,19,23-dimethylheptatriacontane is
Want to see more full solutions like this?
Chapter 10 Solutions
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
- For the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forwardCan I please get this answered? With the correct number of significant digits.arrow_forwardDraw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





