
Concept explainers
(a)
Interpretation:
Lewis structure for a resonance form of each ion of
Concept introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Formula to calculate the formal charge of the atom is as follows:
The formula to calculate the oxidation number of an atom is as follows:
(a)
Answer to Problem 10.17P
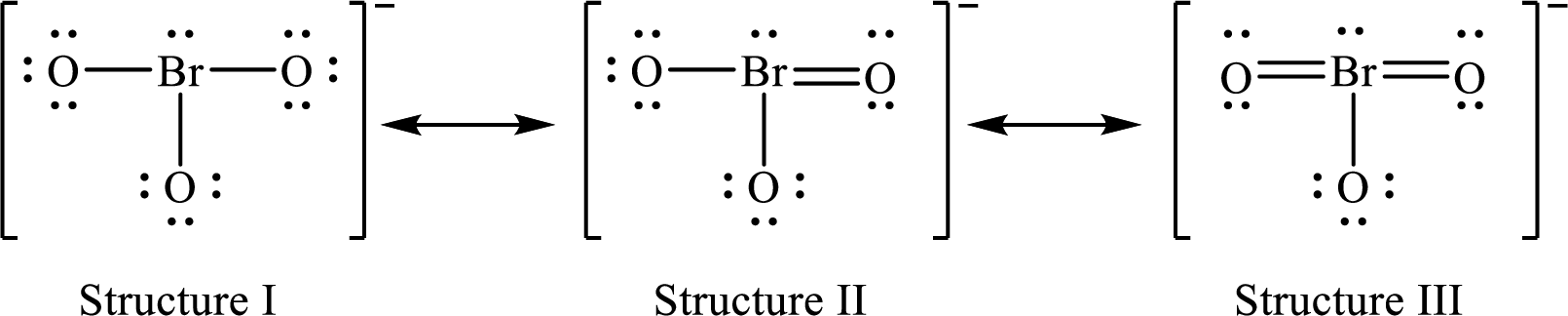
The possible Lewis structures for a resonance form of each ion of
The oxidation numbers of
Explanation of Solution
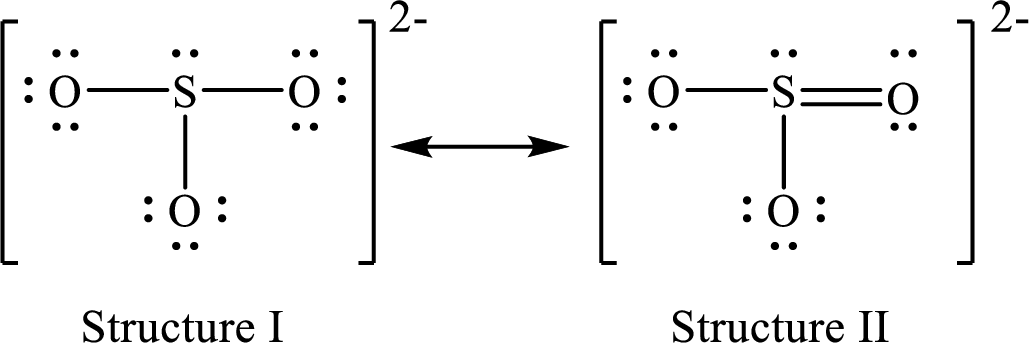
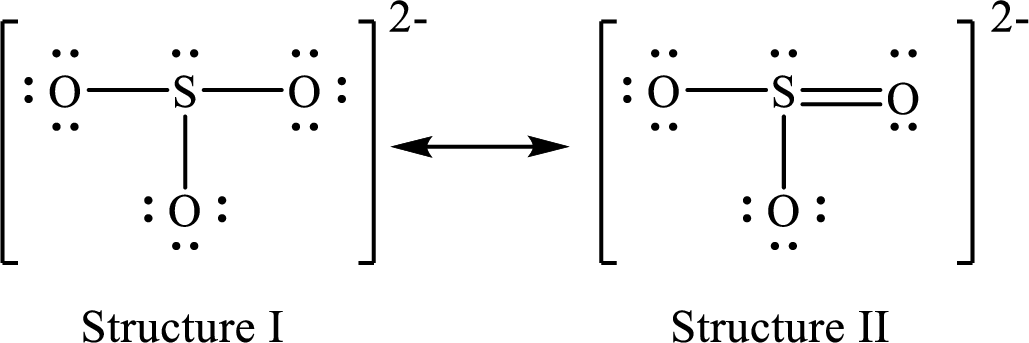
Lewis structures for a resonance form of
For structure I:
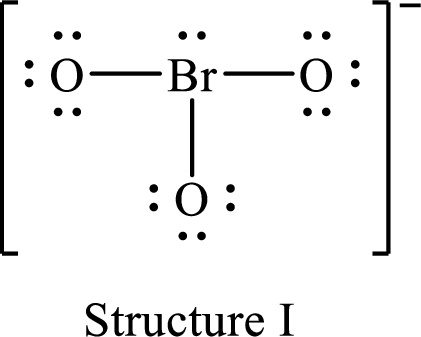
Substitute 7 for valence electrons, 2 for the number of nonbonded electrons and 6 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 6 for nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each oxygen atom.
For structure II:
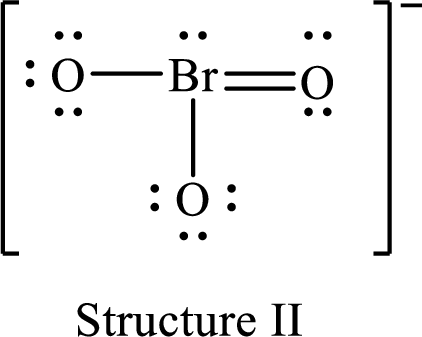
Substitute 7 for valence electrons, 2 for the number of nonbonded electrons and 8 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 6 for nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each single bonded oxygen atom.
Substitute 6 for the value of valence electrons, 4 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on the double bonded oxygen atom.
For structure III:
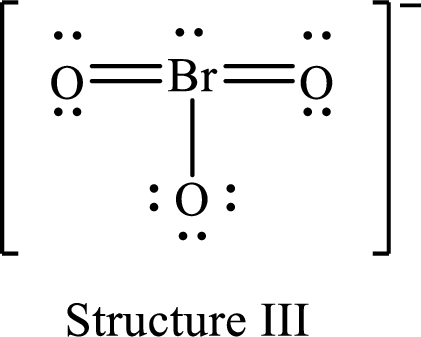
Substitute 7 for valence electrons, 2 for the number of nonbonded electrons and 10 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 6 for nonbonded electrons and 2 for number of bonding electrons in equation (1) to calculate the formal charge on the single bonded oxygen atom.
Substitute 6 for the value of valence electrons, 4 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on each double bonded oxygen atom.
Therefore, structure II has the more acceptable and reasonable distribution of formal charges.
The oxidation numbers of
(b)
Interpretation:
Lewis structure for a resonance form of each ion of
Concept introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Formula to calculate the formal charge of the atom is as follows:
The formula to calculate the oxidation number of an atom is as follows:
(b)
Answer to Problem 10.17P
The possible Lewis structures for a resonance form of each ion of
The oxidation numbers of.
Explanation of Solution
Lewis structure for a resonance form of
For structure I:
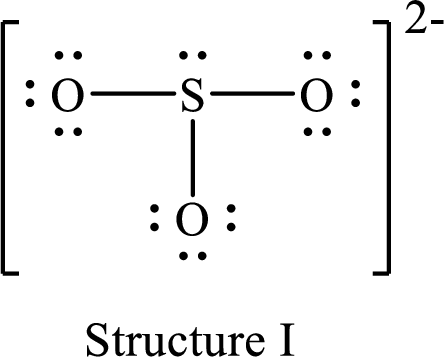
Substitute 6 for valence electrons, 2 for the number of nonbonded electrons and 6 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 6 for nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each oxygen atom.
For structure II:
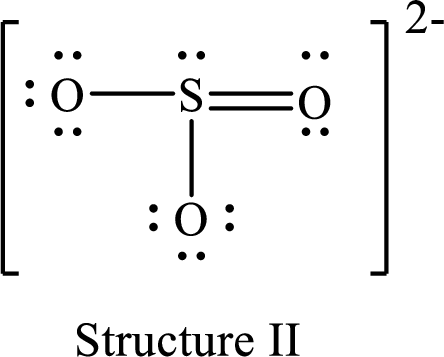
Substitute 6 for valence electrons, 2 for the number of nonbonded electrons and 8 for the number of bonding electrons in equation (1) to calculate the formal charge on
Substitute 6 for valence electrons, 6 for nonbonded electrons and 2 for the number of bonding electrons in equation (1) to calculate the formal charge on each single bonded oxygen atom.
Substitute 6 for the value of valence electrons, 4 for the number of nonbonded electrons and 4 for the number of bonding electrons in equation (1) to calculate the formal charge on the double bonded oxygen atom.
Therefore, structure II has the more acceptable and reasonable distribution of formal charges.
The oxidation numbers of.
Want to see more full solutions like this?
Chapter 10 Solutions
CONNECT ACCESS CARD FOR CHEMISTRY: MOLECULAR NATURE OF MATTER AND CHANGE
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- What would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardWrite the correct IUPAC names of the molecules in the picturearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





