
Loose Leaf for Chemistry
13th Edition
ISBN: 9781260162035
Author: Raymond Chang Dr., Jason Overby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10, Problem 10.16QP
Interpretation Introduction
Interpretation:
- The relationship between the dipole moment and the bond moment has to be discussed.
- The non-polarity of a molecule with bond-moment has to be discussed.
Concept Introduction:
The term dipole moment refers to the quantitative measure of polarity of a bond. It is represented as
For example:
The diatomic molecule
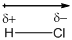
The crossed arrow represents the direction of the shift of electrons towards the highly electronegative chlorine atom form the least electronegative hydrogen atom.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
when performing the reaction that involves 2 equivalents of 3-(diethylamino)-phenol and Phthalic anhydride with sulfuric acid and water react to form rhodamine b where the Phthalic anhydride cleaves in acid and how does Excessive Washing (w/ Base) & Subsequent Resonance Structure get affected
3. The strongest acid of the following compounds is ___.A. p-nitrophenol; B. m-nitrophenol; C. o-chlorophenol;D. p-methoxyphenol; E. o-methylphenol
Please explain your steps and thought process. Thank you!
Using the general properties of equilibrium constants
At a certain temperature, the equilibrium constant K for the following reaction is 1.3 × 10 4:
Cl2(g) + CHCl3(g) HCl(g) + CC₁(g)
Use this information to complete the following table.
Suppose a 16. L reaction vessel is filled with 1.6 mol of HCI and
1.6 mol of CCl4. What can you say about the composition of the
mixture in the vessel at equilibrium?
There will be very little Cl2 and CHCl3.
☐ x10
There will be very little HCI and CCl4.
Neither of the above is true.
What is the equilibrium constant for the following reaction? Be
sure your answer has the correct number of significant digits.
HCl(g)+CC14(g)
12
Cl2(9)+CHCl3(9)
K = 0 ☐
What is the equilibrium constant for the following reaction? Be
sure your answer has the correct number of significant digits.
2 Cl₂(9)+2CHCl3(9)
2 HCl(9)+2CC₁₁(9)
K =
✓
00.
18
Ar
Chapter 10 Solutions
Loose Leaf for Chemistry
Ch. 10.1 - Use the VSEPR model to predict the geometry of (a)...Ch. 10.1 - What is the molecular geometry of GeCl4?Ch. 10.1 - What is the molecular geometry of BrO3?Ch. 10.1 - Which of the following geometries has a greater...Ch. 10.2 - Does the AlCl3 molecule have a dipole moment?Ch. 10.2 - Predict whether PF5 has a dipole moment.Ch. 10.2 - The molecule CF4 does not have a dipole moment...Ch. 10.2 - Carbon dioxide has a linear geometry and is...Ch. 10.3 - Compare the Lewis theory and the valence bond...Ch. 10.4 - Determine the hybridization state of the...
Ch. 10.4 - Describe the hybridization state of Se in SeF6.Ch. 10.4 - How many orbitals does a set of sp3d hybrid...Ch. 10.4 - What is the hybridization of P in PH4+?Ch. 10.4 - What is the hybridization of Xe in XeF4Ch. 10.5 - Describe the bonding in the hydrogen cyanide...Ch. 10.5 - How many pi bonds are present in CS2?Ch. 10.5 - Which of the following pairs of atomic orbitals on...Ch. 10.6 - One way to account for the fact that an O2...Ch. 10.7 - Which of the following species has a longer bond...Ch. 10.7 - Calculate the bond order of F2+.Ch. 10.7 - Determine if N2+ is diamagnetic or paramagnetic.Ch. 10.7 - Estimate the bond enthalpy (kJ/mol) of the H2+...Ch. 10.8 - Describe the bonding in the nitrate ion (NO3) in...Ch. 10 - How is the geometry of a molecule defined and why...Ch. 10 - Sketch the shape of a linear triatomic molecule, a...Ch. 10 - How many atoms are directly bonded to the central...Ch. 10 - Discuss the basic features of the VSEPR model....Ch. 10 - Prob. 10.5QPCh. 10 - Prob. 10.6QPCh. 10 - Predict the geometries of the following species...Ch. 10 - Predict the geometries of the following species:...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometries of the following ions: (a)...Ch. 10 - Describe the geometry around each of the three...Ch. 10 - Which of the following species are tetrahedral?...Ch. 10 - Prob. 10.15QPCh. 10 - Prob. 10.16QPCh. 10 - Prob. 10.17QPCh. 10 - The bonds in beryllium hydride (BeH2) molecules...Ch. 10 - Referring to Table 10.3, arrange the following...Ch. 10 - The dipole moments of the hydrogen halides...Ch. 10 - List the following molecules in order of...Ch. 10 - Does the molecule OCS have a higher or lower...Ch. 10 - Which of the molecules (a) or (b) has a higher...Ch. 10 - Prob. 10.24QPCh. 10 - What is valence bond theory? How does it differ...Ch. 10 - Use valence bond theory to explain the bonding in...Ch. 10 - Prob. 10.27QPCh. 10 - Prob. 10.28QPCh. 10 - Prob. 10.29QPCh. 10 - What is the angle between the following two hybrid...Ch. 10 - Describe the bonding scheme of the AsH3 molecule...Ch. 10 - What is the hybridization state of Si in SiH4 and...Ch. 10 - Describe the change in hybridization (if any) of...Ch. 10 - Consider the reaction BF3+NH3F3BNH3 Describe the...Ch. 10 - What hybrid orbitals are used by nitrogen atoms in...Ch. 10 - Prob. 10.36QPCh. 10 - Give the formula of a cation comprised of iodine...Ch. 10 - Give the formula of an anion comprised of iodine...Ch. 10 - How would you distinguish between a sigma bond and...Ch. 10 - What are the hybrid orbitals of the carbon atoms...Ch. 10 - Specify which hybrid orbitals are used by carbon...Ch. 10 - Prob. 10.42QPCh. 10 - The allene molecule H2CCCH2 is linear (the three C...Ch. 10 - How many pi bonds and sigma bonds are there in the...Ch. 10 - How many sigma bonds and pi bonds are there in...Ch. 10 - What is molecular orbital theory? How does it...Ch. 10 - Sketch the shapes of the following molecular...Ch. 10 - Explain the significance of bond order. Can bond...Ch. 10 - Explain in molecular orbital terms the changes in...Ch. 10 - The formation of H2 from two H atoms is an...Ch. 10 - Prob. 10.51QPCh. 10 - Arrange the following species in order of...Ch. 10 - Prob. 10.53QPCh. 10 - Which of these species has a longer bond, B2 or...Ch. 10 - Acetylene (C2H2) has a tendency to lose two...Ch. 10 - Compare the Lewis and molecular orbital treatments...Ch. 10 - Explain why the bond order of N2 is greater than...Ch. 10 - Compare the relative stability of the following...Ch. 10 - Use molecular orbital theory to compare the...Ch. 10 - A single bond is almost always a sigma bond, and a...Ch. 10 - In 2009 the ion N23 was isolated. Use a molecular...Ch. 10 - The following potential energy curve represents...Ch. 10 - Prob. 10.63QPCh. 10 - Prob. 10.64QPCh. 10 - Prob. 10.65QPCh. 10 - Explain why the symbol on the left is a better...Ch. 10 - Determine which of these molecules has a more...Ch. 10 - Nitryl fluoride (FNO2) is very reactive...Ch. 10 - Describe the bonding in the nitrate ion NO3 in...Ch. 10 - Prob. 10.70QPCh. 10 - Which of the following species is not likely to...Ch. 10 - Draw the Lewis structure of mercury(II) bromide....Ch. 10 - Sketch the bond moments and resultant dipole...Ch. 10 - Although both carbon and silicon are in Group 4A,...Ch. 10 - Acetaminophen is the active ingredient in Tylenol....Ch. 10 - Caffeine is a stimulant drug present in coffee....Ch. 10 - Predict the geometry of sulfur dichloride (SCl2)...Ch. 10 - Antimony pentafluoride, SbF5, reacts with XeF4 and...Ch. 10 - Draw Lewis structures and give the other...Ch. 10 - Predict the bond angles for the following...Ch. 10 - Briefly compare the VSEPR and hybridization...Ch. 10 - Describe the hybridization state of arsenic in...Ch. 10 - Draw Lewis structures and give the other...Ch. 10 - Which of the following molecules and ions are...Ch. 10 - Prob. 10.85QPCh. 10 - The N2F2 molecule can exist in either of the...Ch. 10 - Cyclopropane (C3H6) has the shape of a triangle in...Ch. 10 - The compound 1,2-dichloroethane (C2H4Cl2) is...Ch. 10 - Does the following molecule have a dipole moment?...Ch. 10 - So-called greenhouse gases, which contribute to...Ch. 10 - The bond angle of SO2 is very close to 120, even...Ch. 10 - 3-azido-3-deoxythymidine, shown here, commonly...Ch. 10 - The following molecules (AX4Y2) all have...Ch. 10 - The compounds carbon tetrachloride (CCl4) and...Ch. 10 - Prob. 10.95QPCh. 10 - What are the hybridization states of the C and N...Ch. 10 - Use molecular orbital theory to explain the...Ch. 10 - Referring to the Chemistry in Action essay...Ch. 10 - Which of the molecules (a)(c) are polar?Ch. 10 - Prob. 10.100QPCh. 10 - The stable allotropic form of phosphorus is P4, in...Ch. 10 - Referring to Table 9.4, explain why the bond...Ch. 10 - Use molecular orbital theory to explain the...Ch. 10 - The ionic character of the bond in a diatomic...Ch. 10 - Prob. 10.105QPCh. 10 - Prob. 10.106QPCh. 10 - Aluminum trichloride (AlCl3) is an...Ch. 10 - The molecules cis-dichloroethylene and...Ch. 10 - Prob. 10.109QPCh. 10 - Prob. 10.110QPCh. 10 - The molecule benzyne (C6H4) is a very reactive...Ch. 10 - Assume that the third-period element phosphorus...Ch. 10 - Consider a N2 molecule in its first excited...Ch. 10 - Prob. 10.114QPCh. 10 - Prob. 10.116QPCh. 10 - Draw the Lewis structure of ketene (C2H2O) and...Ch. 10 - TCDD, or 2,3,7,8-tetrachlorodibenzo-p-dioxin, is a...Ch. 10 - Write the electron configuration of the cyanide...Ch. 10 - Prob. 10.120QPCh. 10 - The geometries discussed in this chapter all lend...Ch. 10 - Prob. 10.122QPCh. 10 - Which of the following ions possess a dipole...Ch. 10 - Given that the order of molecular orbitals for NO...Ch. 10 - Shown here are molecular models of SX4 for X = F,...Ch. 10 - Based on what you have learned from this chapter...Ch. 10 - How many carbon atoms are contained in one square...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forwardPredicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward4. The major product from treatment of 2-propanol with the Jonesreagent is ___.A. acetone; B. none of the other answers is correct C. propene; D.propanoic acid; E carbon dioxide. Please include all steps! Thank you!arrow_forward
- 7. All of the following compounds that are at the same oxidation levelare ___.u. methyl epoxide, v. propyne, w. propanal, x. propene,y. 2,2-dihydroxypropane, z. isopropanol?A. u,v,w,y; B. u,v,w; C. v,w,y,z; D. v, z; E. x,y,z Please include all steps. Thank you!arrow_forward9. Which one of the following substituents is the worst leaving group inan SN2 reaction? A. -NH2; B. -OH; C. –F; D. NH3; E. H2O Please include all steps. Thanks!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 2.5 × 105: CO(g) + H2O(g) CO2(g) + H2(g) Use this information to complete the following table. Suppose a 7.0 L reaction vessel is filled with 1.7 mol of CO and 1.7 mol of H2O. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. CO2(9)+H2(g) CO(g)+H₂O(g) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 CO(g)+3H2O(g) = 3 CO2(g)+3H2(g) There will be very little CO and H2O. x10 There will be very little CO2 and H2. 000 Neither of the above is true. K = ☐ K = ☐ 18 Ararrow_forward
- 8. When ethane thiol is treated with hydrogen peroxide the product is___.A. ethane disulfide; B. diethyl sulfide; C. ethane sulfoxide; D. ethanesulfate; E. ethyl mercaptan. Please include all steps. Thanks!arrow_forward5. The major product of the three step reaction that takes place when 1-propanol is treated with strong acid is?A. dipropyl ether; B. propene; C. propanal; D. isopropyl propyl ether;E. 1-hexanol Please include all steps. Thank you!arrow_forward6. The formula of the product of the addition of HCN to benzaldehydeis ___.A. C8H7NO; B. C8H6NO; C. C14H11NO; D. C9H9NO; E. C9H8NO Please include all steps. Thank you!arrow_forward
- Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid K K a name formula name formula nitrous acid HNO2 4.5×10 hydroxylamine HONH2 1.1 × 10 8 hypochlorous acid HCIO 8 3.0 × 10 methylamine CH3NH2 | 4.4 × 10¯ 4 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. 0.1 M KCIO solution PH choose one 0.1 M NaNO2 0.1 M CH3NH3Br 0.1 M NaBr choose one ✓ choose one v ✓ choose one 1 (lowest) ☑ 2 3 4 (highest)arrow_forwardFor this Orgo problem, don't worry about question 3 below it. Please explain your thought process, all your steps, and also include how you would tackle a similar problem. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 0.84: H2(g) + 2(g) 2 HI(g) = Use this information to complete the following table. Suppose a 34. L reaction vessel is filled with 0.79 mol of HI. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little H2 and 12. ☐ x10 There will be very little HI. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 HI(g) H₂(9)+12(9) K = What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 H2(g)+212(9) 4 HI(g) K = ☐ ☑arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY