
Chemistry: A Molecular Approach (4th Edition)
4th Edition
ISBN: 9780134112831
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10, Problem 100E
Interpretation Introduction
Interpretation: The results of a molecular orbital calculation for H2O are shown here. Also, classify each of the as bonding antibonding or nonbonding. According to the following diagram:
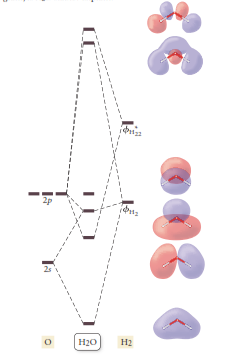
Concept Introduction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q3: Write in the starting alkyl bromide used to form the following products. Include any
reactants, reagents, and solvents over the reaction arrow. If more than one step is
required, denote separate steps by using 1), 2), 3), etc.
H
OH
racemic
OH
OH
5
racemic
Draw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.
Predict all organic product(s), including stereoisomers when applicable.
Chapter 10 Solutions
Chemistry: A Molecular Approach (4th Edition)
Ch. 10 - Prob. 1SAQCh. 10 - Prob. 2SAQCh. 10 - Prob. 3SAQCh. 10 - Prob. 4SAQCh. 10 - Prob. 5SAQCh. 10 - Prob. 6SAQCh. 10 - Prob. 7SAQCh. 10 - Prob. 8SAQCh. 10 - Prob. 9SAQCh. 10 - Q10. Apply molecular orbital theory to predict...
Ch. 10 - Q11. Apply molecular orbital theory to determine...Ch. 10 - Q12. Which hybridization scheme occurs about...Ch. 10 - Q13. Which molecular geometry results when a...Ch. 10 - Prob. 14SAQCh. 10 - Prob. 15SAQCh. 10 - 1. Why is molecular geometry important? Cite some...Ch. 10 - 2. According to VSEPR theory, what determines the...Ch. 10 - 3. Name and sketch the five basic electron...Ch. 10 - 4. Explain the difference between electron...Ch. 10 - 5. Give the correct electron and molecular...Ch. 10 - 6. How do you apply VSEPR theory to predict the...Ch. 10 - Prob. 7ECh. 10 - Prob. 8ECh. 10 - 9. In valence bond theory, what determines the...Ch. 10 - 10. In valence bond theory, the interaction energy...Ch. 10 - Prob. 11ECh. 10 - Prob. 12ECh. 10 - 13. How is the number of hybrid orbitals related...Ch. 10 - Prob. 14ECh. 10 - Prob. 15ECh. 10 - 16. Name the hybridization scheme that corresponds...Ch. 10 - Prob. 17ECh. 10 - Prob. 18ECh. 10 - 19. What is a bonding molecular orbital?
Ch. 10 - 20. What is an antibonding molecular orbital?
Ch. 10 - 21. What is the role of wave interference in...Ch. 10 - Prob. 22ECh. 10 - 23. How is the number of molecular orbitals...Ch. 10 - 24. Sketch each molecular orbital.
a. σ2s
b.
c....Ch. 10 - Prob. 25ECh. 10 - Prob. 26ECh. 10 - Prob. 27ECh. 10 - Prob. 28ECh. 10 - Prob. 29ECh. 10 - 30. Write a short paragraph describing chemical...Ch. 10 - 31. A molecule with the formula AB3 has a trigonal...Ch. 10 - 32. A molecule with the formula AB3 has a trigonal...Ch. 10 - 33. For each molecular geometry, list the number...Ch. 10 - Prob. 34ECh. 10 - 35. Determine the electron geometry, molecular...Ch. 10 - 36. Determine the electron geometry, molecular...Ch. 10 - 37. Which species has the smaller bond angle, H3O...Ch. 10 - Prob. 38ECh. 10 - 39. Determine the molecular geometry and sketch...Ch. 10 - Prob. 40ECh. 10 - Prob. 41ECh. 10 - Prob. 42ECh. 10 - 43. Each ball-and-stick model shows the electron...Ch. 10 - 44. Each ball-and-stick model shows the electron...Ch. 10 - 45. Determine the geometry about each interior...Ch. 10 - Prob. 46ECh. 10 - Prob. 47ECh. 10 - Prob. 48ECh. 10 - Prob. 49ECh. 10 - Prob. 50ECh. 10 - 51. Determine whether each molecule is polar or...Ch. 10 - Prob. 52ECh. 10 - 53. The valence electron configurations of several...Ch. 10 - 54. The valence electron configurations of several...Ch. 10 - 55. Write orbital diagrams (boxes with arrows in...Ch. 10 - Prob. 56ECh. 10 - 57. Write orbital diagrams (boxes with arrows in...Ch. 10 - Prob. 58ECh. 10 - 59. Which hybridization scheme allows the...Ch. 10 - Prob. 60ECh. 10 - Prob. 61ECh. 10 - 62. Write a hybridization and bonding scheme for...Ch. 10 - Prob. 63ECh. 10 - 64. Write a hybridization and bonding scheme for...Ch. 10 - 65. Write a hybridization and bonding scheme for...Ch. 10 - Prob. 66ECh. 10 - 67. Consider the structure of the amino acid...Ch. 10 - 68. Consider the structure of the amino acid...Ch. 10 - 69. Sketch the bonding molecular orbital that...Ch. 10 - Prob. 70ECh. 10 - 71. Draw an MO energy diagram and predict the bond...Ch. 10 - Prob. 72ECh. 10 - Prob. 73ECh. 10 - Prob. 74ECh. 10 - Prob. 75ECh. 10 - 76. Using the molecular orbital energy ordering...Ch. 10 - 77. Use molecular orbital theory to predict if...Ch. 10 - 78. Use molecular orbital theory to predict if...Ch. 10 - Prob. 79ECh. 10 - Prob. 80ECh. 10 - 81. Draw an MO energy diagram for CO. (Use the...Ch. 10 - Prob. 82ECh. 10 - 83. For each compound, draw the Lewis structure,...Ch. 10 - 84. For each compound, draw the Lewis structure,...Ch. 10 - 85. Amino acids are biological compounds that link...Ch. 10 - 86. The genetic code is based on four different...Ch. 10 - 87. The structure of caffeine, present in coffee...Ch. 10 - 88. The structure of acetylsalicylic acid...Ch. 10 - 89. Most vitamins can be classified as either fat...Ch. 10 - 90. Water does not easily remove grease from...Ch. 10 - Prob. 91ECh. 10 - Prob. 92ECh. 10 - 93. Bromine can form compounds or ions with any...Ch. 10 - 94. The compound C3H4 has two double bonds....Ch. 10 - Prob. 95ECh. 10 - Prob. 96ECh. 10 - Prob. 97ECh. 10 - 98. Indicate which orbitals overlap to form the s...Ch. 10 - 99. In VSEPR theory, which uses the Lewis model to...Ch. 10 - 100. The results of a molecular orbital...Ch. 10 - 101. The results of a molecular orbital...Ch. 10 - Prob. 102ECh. 10 - Prob. 103ECh. 10 - Prob. 104ECh. 10 - Prob. 105ECh. 10 - 106. Neither the VSEPR model nor the hybridization...Ch. 10 - 107. Draw the Lewis structure for acetamide...Ch. 10 - Prob. 108ECh. 10 - 109. Which statement best captures the fundamental...Ch. 10 - 110. Suppose that a molecule has four bonding...Ch. 10 - 111. How does each of the three major bonding...Ch. 10 - Prob. 112ECh. 10 - Prob. 113QGWCh. 10 - Prob. 114QGWCh. 10 - Prob. 115QGWCh. 10 - Prob. 116QGWCh. 10 - Prob. 117QGWCh. 10 - Prob. 118DIA
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forwardConsider the solubility products (Ksp values) for the following compounds:SrSO4 (Ksp = 7.6 x 10−7), BaSO4 (Ksp = 1.5 x 10−9), SrCO3 (Ksp = 7.0 x 10−10), BaCO3 (Ksp = 1.6 x 10−9)Which anion is the harder base, CO32− or SO42−? Justify your answer.arrow_forwardQ1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward
- (4 pts - 2 pts each part) A route that can be taken to prepare a hydrophobic (water-repellent) aerogel is to start with trichloromethylsilane, CH3SiCl3 as the silicon source. a. What is the chemical reaction that this undergoes to form a product with Si-OH groups? Write as complete of a chemical equation as you can. CI CI-SI-CH3 CI b. The formation of a byproduct is what drives this reaction - what is the byproduct (if you didn't already answer it in part (a)) and how/why does it form?arrow_forwardb) Circle the substrate that would not efficiently generate a Grignard reagent upon reaction with Mg in ether. CI Br ד c) Circle the Grignard reagents that contain incompatible functional groups. MgBr HO MgBr MgBr MgBr MgBr HO MgBrarrow_forwardQ2: Predict all organic product(s), including stereoisomers when applicable. PCC OH a) CH2Cl2 Page 2 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) .OH Na2Cr2O7, H+ OH PCC CH2Cl2 c) OHarrow_forward
- d) Circle the substrates that will give an achiral product after a Grignard reaction with CH3MgBr. Harrow_forwardQ4: Predict the organic products for the following reactions. Then draw curved arrow electron- pushing mechanism for the reactions. a) NaBH4 EtOH Page 4 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) 요 1. Et₂O H MgBr 2. H+, H₂Oarrow_forward5Helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY