
PRINCIPLES+REACTIONS
8th Edition
ISBN: 9781337759632
Author: Masterton
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 71QAP
Consider the following solubility graph.
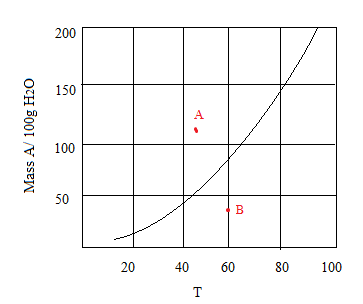
(a) At point A, how many grams of the compound are dissolved in 100 g of water? Is the solution saturated, unsaturated, or supersaturated?
(b) At point B, how many grams of the compound are dissolved in 100 g of water? Is the solution saturated, unsaturated, or supersaturated?
(c) How would you prepare a saturated solution at 30°C?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
10.
Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the
following compound. In order to recieve full credit, you MUST SHOW YOUR WORK!
H₂N
CI
OH
CI
カー
11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign
R/S configurations for all stereoisomers and indicate the relationship between each as
enantiomer, diastereomer, or meso.
NH2
H
HNH,
-18
b)
8.
Indicate whether the following carbocation rearrangements are likely to occur
Please explain your rational using 10 words or less
not likely to occur
• The double bond is still in the
Same position
+
Likely
to oc
occur
WHY?
-3
H3C
Brave
Chair Conformers. Draw the chair conformer of the following substituted
cyclohexane. Peform a RING FLIP and indicate the most stable
conformation and briefly explain why using 20 words or less.
CI
2
-cobs ??
MUST INDICATE H -2
-2
Br
EQ
Cl
OR
AT
Br
H&
most stable
WHY?
- 4
CH
12
Conformational Analysis. Draw all 6 conformers (one above each letter) of the
compound below looking down the indicated bond. Write the letter of the
conformer with the HIGHEST and LOWEST in energies on the lines provided.
NOTE: Conformer A MUST be the specific conformer of the structure as drawn below
-4 NOT
HOH
OH
3
Conformer A:
Br
OH
A
Samo
Br H
04
Br
H
H3
CH₂
H
anti
stagere
Br CH
clipsed
H
Brott
H
IV
H
MISSING 2
-2
B
C
D
E
F
X
6
Conformer with HIGHEST ENERGY:
13. (1
structure
LOWEST ENERGY:
Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the
corresponding to this name. HINT: Do not forget to indicate stereochemistry
when applicable.
a)
८८
2
"Br
{t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa
Parent name (noname)
4 Bromo
Sub = 2-methylethyl-4 Bromo nonane
b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane
# -2
-2
Chapter 1 Solutions
PRINCIPLES+REACTIONS
Ch. 1 - Classify each of the following as element,...Ch. 1 - Classify each of the following as element,...Ch. 1 - Classify the following as solution or...Ch. 1 - Classify the following as solution or...Ch. 1 - Prob. 5QAPCh. 1 - Prob. 6QAPCh. 1 - Write the symbol for the following elements. (a)...Ch. 1 - Prob. 8QAPCh. 1 - Write the name of the element represented by the...Ch. 1 - Write the name of the element represented by the...
Ch. 1 - What instrument would you use to determine (a) the...Ch. 1 - What instrument would you use to (a) measure the...Ch. 1 - A glass of lukewarm milk is suggested for people...Ch. 1 - A recipe for apple pie calls for a preheated 350F...Ch. 1 - Gallium is one of the few metals that can melt at...Ch. 1 - Computers are not supposed to be in very warm...Ch. 1 - How many significant figures are in each of the...Ch. 1 - How many significant figures are there in each of...Ch. 1 - Round off the following quantities to the...Ch. 1 - Round off the following quantities to the...Ch. 1 - Prob. 21QAPCh. 1 - Prob. 22QAPCh. 1 - Which of the following statements use only exact...Ch. 1 - Which of the following statements use only exact...Ch. 1 - A basketball game at the University of...Ch. 1 - A listing of a house for sale states that there...Ch. 1 - Calculate the following to the correct number of...Ch. 1 - Perform the indicated calculations. Write your...Ch. 1 - Prob. 29QAPCh. 1 - The volume of a square pyramid is (1/3)Bh where B...Ch. 1 - Write the appropriate symbol in the blank (,,or=)....Ch. 1 - Write the appropriate symbol in the blank (,,or=)....Ch. 1 - Convert 22.3 mL to (a) liters (b) in3 (c) quartsCh. 1 - Convert 0.2156 L to (a) mL (b) in3 (c) quartsCh. 1 - The height of a horse is usually measured in...Ch. 1 - At sea, distances are measured in nautical miles...Ch. 1 - The unit of land measure in the English system is...Ch. 1 - A gasoline station in Manila, Philippines, charges...Ch. 1 - A lap in most tracks in the United States is 0.25...Ch. 1 - Cholesterol in blood is measured in milligrams of...Ch. 1 - Prob. 41QAPCh. 1 - The area of the 48 contiguous states is 3.02106...Ch. 1 - Prob. 43QAPCh. 1 - In the old pharmaceutical system of measurements,...Ch. 1 - The cup is a measure of volume widely used in...Ch. 1 - The egg whites from four large eggs occupy a...Ch. 1 - A metal slug weighing 25.17 g is added to a flask...Ch. 1 - A solid with an irregular shape and a mass of 11.3...Ch. 1 - A waterbed filled with water has the dimensions...Ch. 1 - Wire is often sold in pound spools according to...Ch. 1 - Air is 21% oxygen by volume. Oxygen has a density...Ch. 1 - The unit for density found in many density tables...Ch. 1 - Magnesium sulfate (MgSO4) has a solubility of 38.9...Ch. 1 - Potassium sulfate has a solubility of 15 g/ 100 g...Ch. 1 - Sodium bicarbonate (baking soda) is commonly use...Ch. 1 - Magnesium chloride is an important coagulant used...Ch. 1 - The solubility of lead nitrate at 100C is 140.0...Ch. 1 - Radiation exposure to human beings is usually...Ch. 1 - The following data refer to the element...Ch. 1 - A supersaturated sugar solution (650.0 g sugar in...Ch. 1 - The density of wind-packed snow is estimated to be...Ch. 1 - The dimensions of aluminum foil in a box for sale...Ch. 1 - The Kohinoor Diamond (d=3.51g/cm3) is 108 carats....Ch. 1 - A pycnometer is a device used to measure density....Ch. 1 - Prob. 65QAPCh. 1 - Label each of the properties of iodine as...Ch. 1 - How do you distinguish (a) chemical properties...Ch. 1 - What is the difference between (a) mass and...Ch. 1 - Mercury, ethyl alcohol, and lead are poured into a...Ch. 1 - How many significant figures are there in the...Ch. 1 - Consider the following solubility graph. (a) At...Ch. 1 - Given the following solubility curves, answer the...Ch. 1 - A Different civilization on a distant planet has...Ch. 1 - At what point is the temperature in F exactly...Ch. 1 - Prob. 75QAPCh. 1 - A laboratory experiment requires 12.0 g of...Ch. 1 - An average adult breathes about 8.50103 L of air...Ch. 1 - A student determines the density of a metal by...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY