
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
8th Edition
ISBN: 9780134581064
Author: Bruice, Paula Yurkanis
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 69P
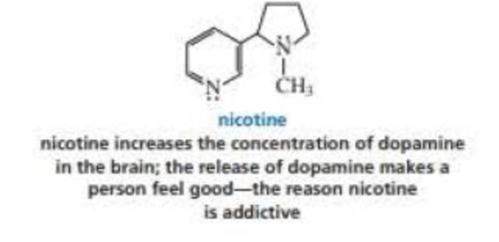
In which orbitals are the lone pairs in nicotine?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
CH3
H+
NaHB(OAc)3
CH3
ZHN.
میں تلاس
e.
N3
.Ph
H+
C.
Br
OH
1. KCN
2. H3O+
Chapter 1 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
Ch. 1.1 - Oxygen has three isotopes, 16O, 17O, and 18O. The...Ch. 1.1 - a. How many protons do the following species...Ch. 1.1 - Chlorine has two isotopes, 35Cl and 37Cl; 75.77%...Ch. 1.2 - Prob. 4PCh. 1.2 - a. Write the ground-state electronic configuration...Ch. 1.2 - Look at the relative positions of each pair of...Ch. 1.3 - a. Find potassium (K) in the periodic table and...Ch. 1.3 - Which bond is more polar? a. b. c. d.Ch. 1.3 - Which of the following has a. the most polar bond?...Ch. 1.3 - Use the symbols + and to show the direction of...
Ch. 1.3 - Explain why HCL has a smaller dipole moment than...Ch. 1.3 - After examining the potential maps for LiH, HF,...Ch. 1.4 - An atom with a formal charge does not necessarily...Ch. 1.4 - Prob. 16PCh. 1.4 - a. Draw two Lewis structure for C2H6O. b. Draw...Ch. 1.4 - Draw the lone-pair electrons that are not shown in...Ch. 1.4 - Prob. 20PCh. 1.4 - Which of the atoms in the molecular models in...Ch. 1.4 - Prob. 22PCh. 1.4 - Prob. 23PCh. 1.5 - Draw the following orbitals: a. 3s orbital b. 4s...Ch. 1.6 - Prob. 25PCh. 1.6 - Indicate the kind of molecular orbital (, , , or )...Ch. 1.7 - What orbitals are used to form the 10 sigma bonds...Ch. 1.7 - Explain why a bond formed by overlap of s orbital...Ch. 1.9 - Put n number in each of the blanks: a. __ s...Ch. 1.9 - For each of the given species: a. Draw its Lewis...Ch. 1.11 - Predict the approximate bond angles in a. the...Ch. 1.11 - According to the potential map for the ammonium...Ch. 1.12 - Prob. 35PCh. 1.13 - a. What are the relative lengths and strengths of...Ch. 1.13 - Prob. 38PCh. 1.14 - Describe the orbitals used in bonding and the bond...Ch. 1.15 - Which of the bond in a carbon-oxygen double bond...Ch. 1.15 - Would you expect a CC bond formed by sp2sp2...Ch. 1.15 - Caffeine is a natural insecticide found in the...Ch. 1.15 - a. What is the hybridization of each of the carbon...Ch. 1.15 - Predict the approximate bond angles for a. the CNC...Ch. 1.16 - What of the following molecules would you expect...Ch. 1.16 - Account for the difference in the shape and color...Ch. 1.16 - If the dipole moment of CH3F is 1.847 D and the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 50PCh. 1 - What is the hybridization of all the atoms (other...Ch. 1 - Draw the condensed structure of a compound that...Ch. 1 - Predict the approximate bond angles: a. the CNH...Ch. 1 - Prob. 54PCh. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - What is the hybridization of each of the carbon...Ch. 1 - Rank the bonds from most polar. a. CO, CF, CN b....Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 59PCh. 1 - What is the hybridization of the indicated atom in...Ch. 1 - Predict the approximate bond angles for the...Ch. 1 - Prob. 62PCh. 1 - Draw the missing lone-pair electrons and assigns...Ch. 1 - a. Which of the indicated bonds in each molecule...Ch. 1 - For each of the following molecules, indicate the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 67PCh. 1 - Rank the following compounds from highest dipole...Ch. 1 - In which orbitals are the lone pairs in nicotine?Ch. 1 - Prob. 70PCh. 1 - Prob. 71PCh. 1 - a. Which of the species have bond angles of 109.5?...Ch. 1 - Prob. 73PCh. 1 - Which compound has a larger dipole moment: CH3Cl...Ch. 1 - Prob. 75PCh. 1 - Explain why CH3Cl has a greater dipole moment than...Ch. 1 - a. Draw a Lewis structure for each of the...Ch. 1 - There are three isomers with molecular formula...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. H3C Br .CH3 H3C NH2arrow_forwarda. OH H₂N-O -Ph H+ acyclic productarrow_forwardeks.com/aleksogi/x/sl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvTCeeBZbufuBYTI0Hz7m7D3ZS17Hd6m-HIl6n52njJN-TXdQA2X9yID-1SWQJTgnjARg30 111 States of Matter Understanding conceptual components of the enthalpy of solution 0/5 Ge A small amount of acetonitrile (CH, CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketched below. (These sketches show only a portion of the substances, so you can see the density and distribution of atoms and molecules in them.) CH,CN H₂O B 88 C Use these sketches to answer the questions in the table below. The enthalpy of solution AH is negative soln when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy. Would heat be absorbed or released if the system moved from Stage C to D? What force would oppose or favor the system moving from Stage C to D? Check all that apply. 1 absorbed O released neither absorbed nor released. none O ionic bonding force covalent bonding force…arrow_forward
- In a system with an anodic overpotential, the variation of ŋ as a function of the current density: 1. at low fields is linear 2. at higher fields, it follows Tafel's law Find the range of current densities for which the overpotential has the same value as when calculated for cases 1 and 2 (maximum relative difference of 5% with respect to the behavior for higher fields). To which overpotential range does this correspond? Data: 10 = 1.5 mA cm², T = 300°C, ẞ = 0.64, R = 8.314 J K 1 mol¹ and F = 96485 C mol-1.arrow_forwardIndicate 10.6 with only one significant figure.arrow_forwardIf I have 10 data points for variables x and y, when I represent y versus x I obtain a line with the equation y = mx + b. Is the slope m equal to dy/dx?arrow_forward
- The data for the potential difference of a battery and its temperature are given in the table. Calculate the entropy change in J mol-1 K-1 (indicate the formulas used).Data: F = 96485 C mol-1arrow_forwardIn a cell, the change in entropy (AS) can be calculated from the slope of the E° vs 1/T graph. The slope is equal to -AS/R, where R is the gas constant. Is this correct?arrow_forwardUsing the Arrhenius equation, it is possible to establish the relationship between the rate constant (k) of a chemical reaction and the temperature (T), in Kelvin (K), the universal gas constant (R), the pre-exponential factor (A) and the activation energy (Ea). This equation is widely applied in studies of chemical kinetics, and is also widely used to determine the activation energy of reactions. In this context, the following graph shows the variation of the rate constant with the inverse of the absolute temperature, for a given chemical reaction that obeys the Arrhenius equation. Based on the analysis of this graph and the concepts acquired about the kinetics of chemical reactions, analyze the following statements: I. The activation energy (Ea) varies with the temperature of the system. II. The activation energy (Ea) varies with the concentration of the reactants. III. The rate constant (K) varies proportionally with temperature. IV. The value of the…arrow_forward
- In an electrolytic cell, indicate the formula that relates E0 to the temperature T.arrow_forward-- 14:33 A Candidate Identification docs.google.com 11. Compound A can transform into compound B through an organic reaction. From the structures below, mark the correct one: HO A تھے۔ די HO B ○ A) Compounds A and B are isomers. B) Both have the same number of chiral carbons. C) Compound A underwent an addition reaction of Cl2 and H2O to form compound B. D) Compound A underwent a substitution reaction forming the intermediate chlorohydrin to obtain compound B. E) Compound A underwent an addition reaction of Cl2 forming the chloronium ion and then added methanol to obtain compound B. 60arrow_forward-- 14:40 A Candidate Identification docs.google.com 13. The compound 1-bromo-hex-2-ene reacts with methanol to form two products. About this reaction, mark the correct statement: OCH3 CH3OH Br OCH3 + + HBr A B A) The two products formed will have the same percentage of formation. B) Product B will be formed by SN1 substitution reaction with the formation of an allylic carbocation. C) Product A will be formed by SN1 substitution reaction with the formation of a more stable carbocation than product B. D) Product A will be formed by an SN2 substitution reaction occurring in two stages, the first with slow kinetics and the second with fast kinetics. E) The two compounds were obtained by addition reaction, with compound B having the highest percentage of formation. 57arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY