
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
8th Edition
ISBN: 2818440043505
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 69P
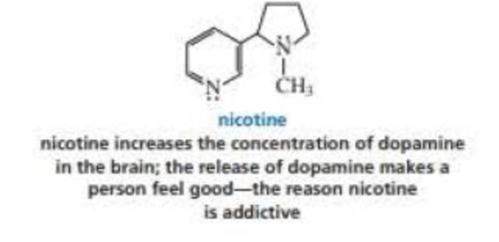
In which orbitals are the lone pairs in nicotine?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products:
xi
1. ☑
2. H₂O
хе
i
Draw the missing reagent X you think will make this synthesis work in the drawing area below.
If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank.
Click and drag to start drawing a
structure.
There is no reagent that will make this synthesis work without complications.
: ☐
S
☐
Predict the major products of this organic reaction:
H
OH
1. LiAlH4
2. H₂O
?
Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry.
Click and drag to start drawing a
structure.
G
C
टे
For each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box.
Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below.
Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first
stable product you expect to form in solution.
NH2
CI
MgCl
?
Will the first product that forms in this reaction
create a new CC bond?
Yes
No
MgBr
?
Will the first product that forms in this reaction
create a new CC bond?
Yes
No
G
टे
Chapter 1 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
Ch. 1.1 - Oxygen has three isotopes, 16O, 17O, and 18O. The...Ch. 1.1 - a. How many protons do the following species...Ch. 1.1 - Chlorine has two isotopes, 35Cl and 37Cl; 75.77%...Ch. 1.2 - Prob. 4PCh. 1.2 - a. Write the ground-state electronic configuration...Ch. 1.2 - Look at the relative positions of each pair of...Ch. 1.3 - a. Find potassium (K) in the periodic table and...Ch. 1.3 - Which bond is more polar? a. b. c. d.Ch. 1.3 - Which of the following has a. the most polar bond?...Ch. 1.3 - Use the symbols + and to show the direction of...
Ch. 1.3 - Explain why HCL has a smaller dipole moment than...Ch. 1.3 - After examining the potential maps for LiH, HF,...Ch. 1.4 - An atom with a formal charge does not necessarily...Ch. 1.4 - Prob. 16PCh. 1.4 - a. Draw two Lewis structure for C2H6O. b. Draw...Ch. 1.4 - Draw the lone-pair electrons that are not shown in...Ch. 1.4 - Prob. 20PCh. 1.4 - Which of the atoms in the molecular models in...Ch. 1.4 - Prob. 22PCh. 1.4 - Prob. 23PCh. 1.5 - Draw the following orbitals: a. 3s orbital b. 4s...Ch. 1.6 - Prob. 25PCh. 1.6 - Indicate the kind of molecular orbital (, , , or )...Ch. 1.7 - What orbitals are used to form the 10 sigma bonds...Ch. 1.7 - Explain why a bond formed by overlap of s orbital...Ch. 1.9 - Put n number in each of the blanks: a. __ s...Ch. 1.9 - For each of the given species: a. Draw its Lewis...Ch. 1.11 - Predict the approximate bond angles in a. the...Ch. 1.11 - According to the potential map for the ammonium...Ch. 1.12 - Prob. 35PCh. 1.13 - a. What are the relative lengths and strengths of...Ch. 1.13 - Prob. 38PCh. 1.14 - Describe the orbitals used in bonding and the bond...Ch. 1.15 - Which of the bond in a carbon-oxygen double bond...Ch. 1.15 - Would you expect a CC bond formed by sp2sp2...Ch. 1.15 - Caffeine is a natural insecticide found in the...Ch. 1.15 - a. What is the hybridization of each of the carbon...Ch. 1.15 - Predict the approximate bond angles for a. the CNC...Ch. 1.16 - What of the following molecules would you expect...Ch. 1.16 - Account for the difference in the shape and color...Ch. 1.16 - If the dipole moment of CH3F is 1.847 D and the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 50PCh. 1 - What is the hybridization of all the atoms (other...Ch. 1 - Draw the condensed structure of a compound that...Ch. 1 - Predict the approximate bond angles: a. the CNH...Ch. 1 - Prob. 54PCh. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - What is the hybridization of each of the carbon...Ch. 1 - Rank the bonds from most polar. a. CO, CF, CN b....Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 59PCh. 1 - What is the hybridization of the indicated atom in...Ch. 1 - Predict the approximate bond angles for the...Ch. 1 - Prob. 62PCh. 1 - Draw the missing lone-pair electrons and assigns...Ch. 1 - a. Which of the indicated bonds in each molecule...Ch. 1 - For each of the following molecules, indicate the...Ch. 1 - Draw a Lewis structure for each of the following:...Ch. 1 - Prob. 67PCh. 1 - Rank the following compounds from highest dipole...Ch. 1 - In which orbitals are the lone pairs in nicotine?Ch. 1 - Prob. 70PCh. 1 - Prob. 71PCh. 1 - a. Which of the species have bond angles of 109.5?...Ch. 1 - Prob. 73PCh. 1 - Which compound has a larger dipole moment: CH3Cl...Ch. 1 - Prob. 75PCh. 1 - Explain why CH3Cl has a greater dipole moment than...Ch. 1 - a. Draw a Lewis structure for each of the...Ch. 1 - There are three isomers with molecular formula...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forwardPredict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forward
- For each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forward
- give example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward
- 3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forwardWhat is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forwardA 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY